The efficacy and safety of oral ritlecitinib (JAK3/TEC inhibitor) and brepocitinib (TYK2/JAK1 inhibitor) were assessed in a 32-week Phase 2b induction-maintenance umbrella study (VIBRATO) in participants with moderate to severe active ulcerative colitis who had inadequate or loss of response, or intolerance to corticosteroids, immunosuppressants, or biologic therapies. We report efficacy and safety results from the 8-week induction period of the VIBRATO study.
Adult participants with Total Mayo Score ≥6 and centrally-read Mayo endoscopic subscore ≥1 were randomised to receive oral ritlecitinib 20, 70, or 200 mg; brepocitinib 10, 30, or 60 mg; or placebo once-daily (QD) for 8 weeks. Participants then continued in their respective treatment cohorts to receive ritlecitinib 50 mg or brepocitinib 30 mg QD for 24 weeks. The proportions of patients who achieved remission (Total Mayo Score ≤2; no individual subscore >1; rectal bleeding subscore 0), modified remission (Modified Mayo Score: Total Mayo without Physician’s Global Assessment; stool frequency subscore ≤1; rectal bleeding subscore 0; endoscopic subscore ≤1), or endoscopic improvement (Mayo endoscopic subscore ≤1) were analysed.
Results319 participants were randomised: baseline mean (standard deviation [SD]) age 40.3 (13.8) years; mean (SD) Total Mayo Score 9.0 (1.5); and median (range) disease duration 4.8 (0.24, 36.5) years. Ritlecitinib and brepocitinib were generally safe and well tolerated. At Week 8, a dose–response relationship was observed across all efficacy endpoints for ritlecitinib and brepocitinib. The proportions of participants achieving remission were significantly higher (P<0.05) with ritlecitinib 70 and 200 mg and brepocitinib 30 and 60 mg vs placebo (Figure 1). The proportions of participants achieving endoscopic improvement and modified remission were significantly higher in all ritlecitinib and brepocitinib groups vs placebo (Figures 2 and 3).



Ritlecitinib 70 and 200 mg QD and brepocitinib 30 and 60 mg QD demonstrated significant improvement in remission, modified remission, and endoscopic improvement in participants with moderate to severe active ulcerative colitis.
AJM300 (INN; carotegrast methyl), an orally active small molecule antagonist of the α4 subunit of α4β1/α4β7 integrins, demonstrated the efficacy and safety in patients with moderately active ulcerative colitis (UC) in a phase 2 study. The phase 3 study (NCT 03531892) of AJM300 as induction therapy was conducted in patients with moderately active UC.
MethodsEligible patients were moderately active Japanese UC, defined as total Mayo Clinic scores (MCS) of 6-10, endoscopic subscores (ES) ≥2, and rectal bleeding subscores (RBS) ≥1, who had inadequate response or intolerance to oral 5-ASA. Followed by a 2-week single-blind placebo (PBO) run-in phase, patients were randomized 1:1 to receive AJM300 960 mg or PBO 3 times daily for 8 weeks. Responders or remitters were allowed to receive AJM300 960 mg again at the subsequent relapse (open-label). The primary endpoint was clinical response at week 8, defined as reduction of the MCS of ≥3-pts and ≥30%, reduction in RBS of ≥1-pt or RBS of ≤1, and ES ≤1.
ResultsThe randomized 203 patients had moderately active endoscopic evidence at baseline with median UC duration of 6.1 years and MCS of 7.8. For the primary endpoint, 45.1% (46/102) and 20.8% (21/101) of patients in the AJM300 and PBO groups, respectively, achieved clinical response at week 8 (OR=3.30 [95% CI, 1.73-6.29]; p=0.0003). Symptomatic remission, endoscopic improvement and endoscopic remission were also statistically significant for AJM300 vs PBO (Table). In case of episodic AJM300 treatment, AJM300 exhibited similar response to initial treatment. Overall, the incidence of AEs and serious AEs were similar between AJM300 and PBO. There were no deaths or cases of progressive multifocal leukoencephalopathy.
AJM300 induced clinical response as well as endoscopic remission with good tolerability. AJM300 may become a novel therapeutic option for patients who had inadequate response or intolerance to oral 5-ASA.
Table. Efficacy results at Week 8
| Endpoint | PBO, n (%) (n=101) | AJM300, n (%) (n=102) | Percent difference (95% CI) | P value |
| Clinical response | 21 (20.8) | 46 (45.1) | 24.3 (11.4,36.1) | 0.0003 |
| Clinical remission | 14 (13.9) | 23 (22.5) | 8.7 (-2.0,19.2) | 0.1089 |
| Symptomatic remission | 22 (21.8) | 42 (41.2) | 19.4 (6.6,31.3) | 0.0029 |
| Endoscopic improvement | 27 (26.7) | 56 (54.9) | 28.2 (14.7,40.2) | <0.0001 |
| Endoscopic remission | 3 (3.0) | 14 (13.7) | 10.8 (3.1,19.0) | 0.0057 |
Clinical response=a reduction of the MCS of ≥3-pts and ≥30%, reduction in RBS of ≥1-pt or RBS of ≤1, and ES ≤1; Clinical remission=MCS≤2 and no subscores >1; Symptomatic remission=total of RBS and stool frequency subscores ≤1; Endoscopic improvement=ES ≤1; Endoscopic remission=ES =0.
CI, confidence interval; ES, endoscopic subscores; MCS, Mayo Clinic Score; PBO, placebo; RBS, rectal bleeding subscores.
The STARDUST study demonstrated that ustekinumab (UST), using either a treat-to-target (T2T) or standard of care (SoC) strategy, may induce and maintain endoscopic and clinical response and remission in Crohn’s disease (CD). Primary endpoint, safety, and efficacy have been reported previously.1 Because corticosteroid (CS) sparing is an important aim of CD management, we compared the efficacy of UST T2T vs SoC in achieving CS-free clinical remission and endoscopic response.
MethodsAdult patients (pts) with moderate–severely active CD who were CDAI 70 responders after 16 weeks (W) of induction, comprising a single dose of UST 6 mg/kg iv followed by UST 90 mg SC at W8, were randomized to either T2T or SoC arms. In the T2T arm, choice of UST maintenance dosage (q12w or q8w) was based on endoscopic improvement at W16, followed by clinical and biomarker-directed dose escalation up to q4w; in the SoC arm, UST q12w or q8w dosage was based on EU SmPC. Primary endpoint was endoscopic response (Simple Endoscopic Score in CD [SES-CD] decrease from baseline [BL] ≥50%) at W48. For pts on CS at W16, CS tapering was mandatory. At W48, CS-free clinical remission (CDAI <150 and no CS for ≥30 days) and CS-free endoscopic response (reduction from BL in SES-CD ≥50% and no CS for ≥30 days) were evaluated.
ResultsOf 500 pts enrolled, 441 achieved a CDAI 70 response at W16 and were randomized to T2T (n=220) or SoC (n=221); 79.1% and 87.3%, respectively, completed W48. Among clinical remitters and responders at W16 (start of CS tapering), in both T2T and SoC arms more than 70% were still in remission or response at W48 (Figure 1). CS use throughout 48 weeks of treatment is summarized in Table 1. At W48, in T2T and SoC arms similar rates were noted for CS-free endoscopic response (33.6% and 28.5%, respectively) and CS-free clinical remission (56.4% and 63.3%, respectively). Notably, in T2T and SoC arms the CS-free clinical remission rate among pts on CS at BL was 44.1% and 45.1%, respectively (Figure 2). Among W48 endoscopic responders (T2T, n=83; SoC, n=66), CS-free endoscopic response rate was 89.2% and 95.5%, respectively; among W48 clinical remitters (T2T, n=135; SoC, n=154), CS-free clinical remission rate was 91.9% and 90.9%, in T2T and SoC arms, respectively.


Pts treated with UST under T2T or SoC strategies achieved similar rates of CS-free clinical remission and endoscopic response over 48 weeks. Overall for pts on CS at BL, UST reduced the need for CS while achieving response/remission. Most (>89%) pts with endoscopic response/clinical remission at W48 were also CS-free responders/remitters.
1. Danese S, et al. United European Gastroenterol J. 2020;8:1264–1265 (Abstract LB11).
Background
Endoscopic healing has become a critical treatment target in Crohn’s disease (CD). Risankizumab (RZB), a humanized immunoglobulin G1 monoclonal antibody against the p19 subunit of interleukin 23, is being investigated as a treatment for moderate-to-severe CD. This analysis assessed different endoscopic endpoints in patients treated with RZB induction therapy in twodouble-blind, randomised, placebo (PBO)-controlled studies (ADVANCE [NCT03104413] and MOTIVATE [NCT03105128]).
Methods
Patients with moderate-to-severe CD (CD Activity Index [CDAI] of 220–450, Simple Endoscopic Score for CD [SES‑CD] ≥ 6 [≥ 4 for isolated ileal disease] excluding the narrowing component, and average daily [liquid/very soft] stool frequency [SF] ≥ 4 and/or average daily abdominal pain [AP] score ≥ 2) who had demonstrated prior inadequate response or intolerance to conventional and/or biologic treatment (ADVANCE) or to biologic treatment (MOTIVATE) were randomised 2:2:1 (ADVANCE) or 1:1:1 (MOTIVATE) to receive intravenous (IV) RZB 600 mg, RZB 1200 mg, or PBO at weeks 0, 4, and 8. This analysis evaluated the proportion of patients who achieved endoscopic remission ulcer-free endoscopy (ie, absence of ulcers), and composite endpoints of CDAI clinical response and endoscopic response, and enhanced clinical response and endoscopic response at week 12 (endpoints defined in Figure 1 footnotes). All endoscopies were centrally read by a blinded reviewer. Safety was assessed throughout the studies.
Results
In ADVANCE and MOTIVATE, 850 and 569 patients, respectively, were randomised and included in the intent-to-treat population for this analysis. At week 12 greater proportions of RZB- vs PBO-treated patients in both studies achieved endoscopic remission (P ≤ .001), ulcer-free endoscopy (P ≤ .01), CDAI clinical response and endoscopic response (P ≤ .001), and enhanced clinical response and endoscopic response (P ≤ .001; Figure 1). Treatment with RZB 600 mg or 1200 mg was well tolerated, and no new safety risks were identified.1,2

1. D’Haens G et al. ADVANCE study. Abstract presented at Digestive Disease Week 2021; 21-23 May 2021; Virtual.
2. AbbVie In. (7 Jan 2021). Risankizumab (SKYRIZI®) Demonstrates Significant Improvements in Clinical Remission and Endoscopic Response in Two Phase 3 Induction Studies in Patients with Crohn's Disease [Press release]. Retrieved from https://news.abbvie.com/news/press-releases/risankizumab-skyrizi-demonstrates-significant-improvements-in-clinical-remission-and-endoscopic-response-in-two-phase-3-induction-studies-in-patients-with-crohns-disease.htm
Conclusion
Induction therapy with IV RZB 600 mg or 1200 mg resulted in improved outcomes at week 12 compared with PBO as assessed by endoscopy and by composite endoscopic-clinical endpoints in patients with moderate-to-severe CD.
Filgotinib (FIL) is a preferential Janus kinase 1 inhibitor in development for the treatment of inflammatory bowel disease. SELECTION was a phase 2b/3 randomized, double-blind, placebo (PBO)-controlled trial to evaluate FIL for the treatment of moderately to severely active ulcerative colitis (UC) (NCT02914522). The aim of this post hoc analysis was to assess the speed of improvement in patient-reported outcomes (PROs) during FIL treatment.
MethodsEligible patients who were biologic-naïve or -experienced were enrolled in induction study A or induction study B, respectively. In each study, patients were randomized 2:2:1 to receive FIL 100 mg, FIL 200 mg or PBO once daily orally for 10 weeks. In this post hoc analysis, data from daily patient diaries up to day 15 of induction, including Mayo stool frequency subscores (SF; range, 0 [normal] to 3 [≥5 stools/day more than normal]) and rectal bleeding subscores (RB; range, 0 [no blood] to 3 [passing blood alone]), were used to evaluate the proportion of patients achieving predefined subscores or subscore reductions.
ResultsInduction studies A and B comprised 659 and 689 patients, respectively. Baseline characteristics were similar across treatment groups within induction study A and within induction study B. In induction study A, more patients treated with FIL 200 mg vs PBO reported a reduction in SF of ≥1 from baseline as early as day 6 (FIL 200 mg, 35.8%; PBO, 20.6%, p<0.01) and every day from day 10 (Figure 1), and a reduction in RB of ≥1 from baseline as early as day 4 (FIL 200 mg, 36.9%; PBO, 23.7%; p<0.01) and every day from day 7 (Figure 2). In induction study B, more patients treated with FIL 200 mg vs PBO reported a reduction in SF of ≥1 from baseline as early as day 2 (FIL 200 mg, 21.6%; PBO, 12.1%; p<0.05) (Figure 3) and a reduction in RB of ≥1 from baseline as early as day 3 (FIL 200 mg, 29.5%; PBO, 17.6%; p<0.01) (Figure 4). More patients receiving FIL 200 mg vs PBO achieved the composite score of RB=0 and SF≤1 as early as day 9 in induction study A (FIL 200 mg, 18.8%; PBO, 9.5%, p<0.05). More patients receiving FIL 200 mg vs PBO achieved the composite score of RB=0 and SF≤1 as early as day 7 in induction study B (FIL 200 mg, 10.7%; PBO, 4.2%, p<0.05).



In this post hoc analysis of induction study data from SELECTION, improvements in SF and RB were observed within the first week of therapy with FIL 200 mg, compared with PBO, in patients with moderately to severely active UC. These data demonstrate that FIL 200 mg has rapid onset of action, as assessed by PROs, in both biologic-naïve and biologic-experienced patients.
Patients with ulcerative colitis (UC) are enrolled into surveillance programs for the early detection of colorectal cancer (CRC). However, most patients under surveillance are low-risk and never progress to CRC, while a significant proportion of CRCs in UC form without a preceding confirmed diagnosis of dysplasia. High resolution chromosomal copy-number alteration (CNA) analysis of unselected formalin-fixed paraffin embedded biopsies taken at surveillance colonoscopies using low pass whole genome sequencing (lpWGS) offers an appealing approach to CRC stratification.
MethodsWe conducted a retrospective case-control study to compare the CNA burden in four unselected non-neoplastic left-sided colorectal biopsies from patients with E2/E3 UC derived 1-5 years prior to HGD/CRC detection (cases), with that of biopsies from patients who subsequently remained HGD/CRC-free for at least 5 years (controls). The two patient groups were matched by age, gender, duration of IBD and PSC status. lpWGS was performed using a standardised pipeline for epithelial enrichment, DNA extraction, library preparation, next generation sequencing and bioinformatic analysis.
476 biopsies, derived from 42 cases and 77 controls, were analysed. Nearly 80% of patients had a detectable CNA in at least one of their biopsies, with the maximal CNA burden in a typical biopsy involving a median 1.1% of that biopsy’s genome. The CNA burden was significantly greater in the rectum compared to the sigmoid colon and descending colon. The most common CNA events were losses of between 1-30 megabases involving the sub-telomeric regions of chromosomes 5-9 and 22, which were found in similar proportion in both case and control biopsies. However, losses extending beyond sub-telomeric regions, as well as copy number gains, were found more frequently in cases biopsies (p<0.0001). The most discriminating CNA event was the presence of such a loss extending beyond subtelomeric regions in any of the patient’s four biopsies, with a high specificity exceeding 0.95 (see Kaplan-Meier plot). ROC analysis demonstrates that lpWGS output has a fair level of accuracy at predicting future HGD/CRC risk (AUC 0.73). 
We identified multiple biopsies, predominantly in cases, with a surprisingly marked CNA burden involving over 10% of the genome, highlighting the fluid phenotype-genotype relationship. Non-dysplastic colitic epithelium can bear a significant burden of CNAs and maintain phenotypic stability for years without neoplastic transformation. Remarkably, by analysing the CNA burden of only four random biopsies, derived from less than 0.05% of the colonic surface area, we can significantly discriminate between case and control cohorts.
Protein profiling in patients with inflammatory bowel diseases (IBD) for diagnostic and therapeutic purposes is underexplored. Assessment of interactions between genetics and the plasma proteome could lead to identification of novel disease-associated molecular pathways. In this study, we performed the largest gene-protein association analysis thus far in patients with IBD, taking into account relevant phenotypic covariates and integrating information from multiple biological data layers.
MethodsNinety-two (92) inflammation-related proteins were quantified in plasma of 1,028 patients with IBD (567 Crohn’s disease [CD]; 461 ulcerative colitis [UC]) and 148 healthy individuals to assess proteome-phenotype associations. Both whole-exome sequencing (WES) and global screening array (GSA) data of 919 patients with IBD were included to study associations between over 8 million genetic variants and protein levels (protein quantitative trait loci [pQTL]). Cis-pQTLs were defined within ± 1 Mb of the region of each protein-coding gene center, whereas trans-pQTLs were outside of that region. After adjusting for phenotypic covariates, a step-wise conditional analysis was used to identify all independent pQTLs in CD and UC separately, followed by a meta-analysis. Intestinal mucosal RNA sequencing and fecal metagenomic data were used for complementary analyses.
ResultsThirty-four (34) proteins were differentially abundant between IBD and healthy individuals, of which 24 proteins independent of active inflammation. (Figure 1) Seventy-two (72) proteins were significantly associated to 14 phenotypic factors, including age, sex, medication use, and surgical history. (Figure 2) Fibroblast growth factor-19 (FGF-19) levels were decreased in CD patients with ileal disease or a history of ileocecal resection. Thirteen (13) novel cis-pQTL variants were identified and 10 replicated from previous studies, together affecting 21 different plasma proteins. One trans-pQTL variant of the FUT2 gene (rs602662) and two independent cis-pQTL variants of the CCL25 gene significantly affected plasma C-C motif chemokine ligand 25 (CCL25) levels. (Figure 3) Intestinal gene expression data revealed an overlapping cis-expression (e)QTL-variant (rs3745387) of the CCL25 gene. The FUT2 rs602662 trans-pQTL variant associated significantly with reduced abundances of multiple fecal butyrate-producing bacteria, including the genus Blautia and the species Faecalibacterium prausnitzii.


This study shows that both genotype and multiple disease phenotypes strongly associate with the plasma proteome in patients with IBD and identifies disease-associated pathways that may help to improve disease management in the future.
The 48-week (W) interventional STARDUST trial assessed whether a treat-to-target (T2T) strategy using ustekinumab (UST) may optimize Crohn’s disease (CD) outcomes; primary efficacy and safety data have been reported before.1 Here we assessed which patient (pt) subgroups may benefit from T2T vs standard of care (SoC) in achieving endoscopic response after 1 year of UST treatment.
MethodsAdult pts with moderate–severely active CD (CD activity index [CDAI] 220–450) and Simple Endoscopic Score in CD [SES-CD] ≥3) who failed conventional therapy and/or 1 biologic were included. Pts received iv, weight-based UST ~6 mg/kg at W0 (baseline [BL]); then SC UST 90 mg at W8. At W16, CDAI 70 responders were randomized (1:1) to T2T or SoC arms. Pts in the T2T arm were assigned to SC UST q12w or q8w based on 25% improvement in SES-CD score vs BL. From W16–48, UST dose was further intensified up to q4w if the following were not met: CDAI <220 and ≥70-point improvement from BL, and C-reactive protein ≤10 mg/L or faecal calprotectin (FCal) ≤250 µg/g. Pts who failed treatment target despite UST q4w were discontinued. In the SoC arm, UST dose was assigned based on EU SmPC (q12w or q8w). We report the treatment effect for the primary endpoint (endoscopic response [≥50% improvement in SES-CD score vs BL] at W48), evaluated for subgroups of pts, based on demographics at BL. For each subgroup, the odds ratio (OR) and 95% confidence interval (CI) of T2T vs SoC were provided based on the logistic regression model that included treatment arm and stratification factors (prior exposure to biologics [none or 1] and SES-CD score [≤16, >16] at BL) as independent variables.
ResultsOf 500 pts enrolled, 441 were randomized to T2T (n=220) or SoC (n=221); 79.1% and 87.3% completed W48. At W48, pts randomized to T2T were more likely to achieve endoscopic response compared to SoC (p<0.05), if they had at BL: (i) longer disease duration (>median [79.1 months]; OR 2.2; 95%CI 1.17–3.94); (ii) clinically moderate disease (CDAI ≤300; OR 1.7; 95%CI 1.03–2.76); (iii) normal FCal (≤250; OR 3.0; 95%CI 1.22–7.56), (iv) endoscopically active CD (SES-CD ≥4 for ileal or ≥6 for colonic and/or ileocolonic disease; OR 1.8; 95%CI 1.10–2.91); and (v) history or presence of strictures/fistula or occurrence of an intra-abdominal abscess (OR 2.3; 95%CI 1.06–5.01 and OR 3.5; 95%CI 1.07-11.19, respectively; Figure 1).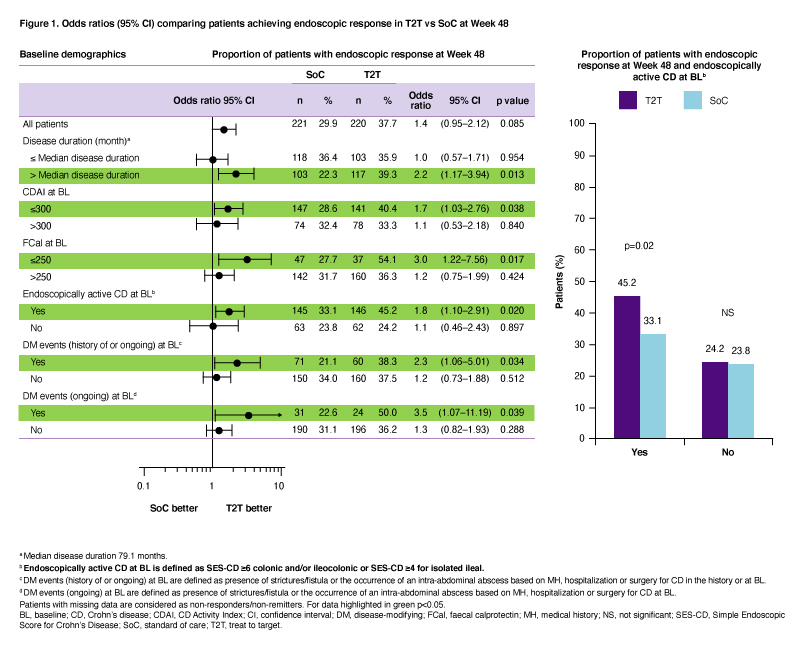
T2T was more effective than SoC (p<0.05) in achieving endoscopic response after 1 year of UST treatment in certain subgroups including pts with higher endoscopic scores at BL and those with history/presence of bowel damage.
1. Danese S, et al. United European Gastroenterol J. 2020;8:1264–1265 (Abstract LB11).
1. To understand available data in orphan fields in IBD
2. To undestand what the factors are hampering data availability and interpretation
3. To explore room for future improvement
Diagnosis of Crohn disease and Ulcerative Colitis is not always easy in biopsy specimens. In this presentation the main features of these diseases are summarized. It is highlighted the importance of multidisciplinary approach and clinical information to classify lesions and to optimize patient management.
Educational objectives:
- to identify the main pathological features of IBD.
- to interpretate the specimen considering clinical-endoscopic information.
To understand the key steps important in optimal macroscopic assessment of IBD specimens
To understand that the priorities of patients may may change and are different to the priorities of physicians.
1. to have an overview over the incidence of proctectomy and perineal sinus in CD
2. to see the evidence on how perineal sinus may be prevented after proctectomy in CD
3. to see the evidence on the surgical treatment of a perineal sinus in CD
4. to understand one possible algorithm of treating a perineal sinus in CD
To understand the importance of perineal ultrasound in the diagnosis and follow up of Crohn's disease
To understand the technical aspects of perineal ultrasound
To understand the features of normal perineal ultrasound and of specifiec pathologies ( Fistula, abscess)
To understand the value of enhanced recovery pathways in improving patients' outcomes
To understand the key elements of succesful implementation of enhanced recovery pathways
1. To overview the current approaches for personalized nutrition in IBD
2. To discuss practical considerations when tailoring dietary recommendations to patients with IBD
2. To discuss challenges and future directions
