A major challenge of future IBD patient care is the definition of actionable disease subphenotypes, e.g. to reliably predict disease progression and to provide a rationale for individual therapy selection. Although advances have been made in the description of genetic risk factors and formal pathophysiology (e.g. the description of inflammatory signal transduction), the diagnostic application of non-standard molecular markers in this setting is rather limited. Many studies have focused on investigating pre-selected sets of cellular or transcriptomic signatures at a single timepoint and thereby do not exploit the dynamic molecular changes that may explain individual disease trajectories.Thus, there is an urgent need for improved longitudinal biomarkers and clinical trial designs, which aim to translate such biomarker sets into actual clinical care . Future long-term strategies will likely include diagnostic features from different molecular ( i.e. omics-) layers. I will review selected pivotal molecular studies and aim to delineate promising clinical questions, which could be solved by collaborative efforts across Europe.
Learning Objectives:
1. Pre conception counseling, optimal disease control, planning, adherence
2. Drug safety at conception and during pregnancy
3. Management of disease exacerbation during pregnancy, assessment and therapeutic options
4. Management of biologics during pregnancy and post-partum
5. Multidisciplinary decision concerning through the entire pregnancy and important decision like mode of delivery
Summary: Therapeutic drug monitoring (TDM) has emerged as a useful tool for optimizing biological therapy, and particularly anti-tumor necrosis factor (anti-TNF) therapy, in patients with inflammatory bowel disease (IBD). Growing evidence suggest that proactive TDM of anti-TNF therapy, with the goal of targeting a predefined drug concentration threshold, is associated with better therapeutic outcomes compared to standard of care. Proactive TDM can also be utilized when infliximab de-escalation is considered and following infliximab re-initiation after a drug holiday. However, there are still several challenges regarding the widespread utilization of proactive TDM in clinical practice including the identification of the optimal drug concentration to target. Future perspectives of proactive TDM of biologics include the implementation of model-informed precision dosing and pharmacogenetics towards personalized medicine.
Educational objectives:
- · To introduce the concept of therapeutic drug monitoring (TDM) of biologics
- · To review the data regarding the role of proactive TDM for optimizing biologics in IBD
- · To describe the potential applications of proactive TDM of biologics in IBD
- · To identify the knowledge gaps regarding utilization of proactive TDM of biologics in IBD clinical practice
- · To discuss the future perspectives of proactive TDM of biologics in IBD
Proactive therapeutic drug monitoring (TDM), individualized treatment based on scheduled assessments of serum drug levels, has been proposed to optimize efficacy and safety of infliximab and other biologic drugs. However, it is unclear whether this strategy improves clinical outcomes.
MethodsIn this 52-week randomised, open-label, multicenter trial, adult patients with an established diagnosis of ulcerative colitis (UC), Crohn’s disease (CD), rheumatoid arthritis (RA), spondyloarthritis (SpA), psoriatic arthritis (PsA), and psoriasis (Ps) receiving infliximab therapy for a minimum of 30 weeks were randomly assigned to proactive TDM or standard infliximab treatment. In the TDM group, infliximab dosage was adjusted according to an algorithm designed to maintain serum infliximab levels within the therapeutic range 3-8 mg/L. In the standard treatment group, infliximab dosage was based on clinical judgement.The primary endpoint was sustained disease control during the 52 week study period.
ResultsIn total, 458 patients were randomised of whom 454 (UC 81, CD 66, RA 79, PsA 53, SpA 138, Ps 37) received the allocated strategy and were included in the primary analyses. The two groups were balanced regarding baseline demographics, clinical and treatment characteristics. Sustained disease control without disease worsening was observed in 167 (73.6 %) patients in the TDM group and in 127 (55.9%) patients in the standard treatment group. The estimated adjusted difference was 17.6% (95% confidence interval (CI), 9.0-26.2, p<0.001), favouring TDM (figure 1). Results were consistent in sensitivity analyses. Time to disease worsening was shorter in the standard treatment group, hazard ratio 2.1 (95% CI 1.5-2.9) (figure 2). Other secondary endpoints reflecting change in disease activity and patient reported outcomes from baseline to week 52 did not show significant differences between the two groups. During the trial, the mean infliximab dose (4.8 mg/kg) and median serum level of infliximab (5.8 mg/L) were comparable in both groups. Twenty-one (9%) patients in the TDM group and 27 (15%) in the standard treatment group developed clinically significant levels of anti-drug antibodies (≥50µg/L). Adverse events were reported in 137 (60%) and 142 (63%) patients in the TDM and standard treatment groups, respectively.
Figure 1

Figure 2
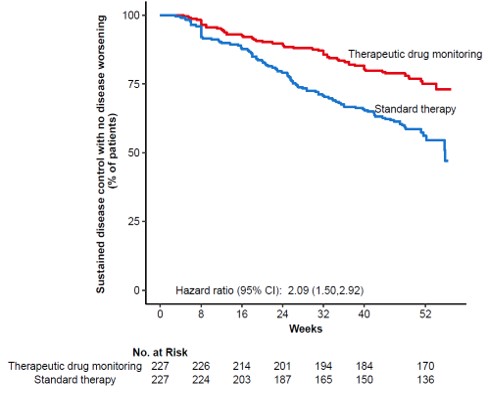
This large randomised controlled trial demonstrates that proactive TDM is superior to standard treatment for maintaining disease control without disease worsening in patients on maintenance therapy with infliximab. These results support implementation of proactive TDM as a general strategy during maintenance therapy with infliximab and have the potential to change clinical practice across specialities.
Perianal manifestation in Crohn’s disease patients is likely to complicate the disease course with extra intestinal manifestations, abscesses, deep anal canal ulcers, luminal fistulas and strictures, steroid resistance, and need for multiple surgeries. Diagnosis and management of perianal Crohn’s disease implies a multidisciplinary team approach. Diagnosis and definition of perianal disease requires optimal imaging modality, ideally a pelvic magnetic resonance imaging, with an exam under anesthesia (EUA). However, the lack of a definition consensus on perianal fistula in Crohn’s disease may affect standardization of therapeutic approaches and patients inclusion within clinical trial.
The synergic approach by a surgeon and a gastroenterologist is crucial with perianal Crohn’s disease. Drainage of an abscess and possible seton placement to prevent future septic complications is the basic first step of the treatemnt. Ani-TNF drug have shown the best evidence for decreasing perianal drainage and promote fistula healing. Attempting surgical repair is possibile for selected patients. Surgical strategies include subcutaneous fistulotomy, Ligation of the Intersphincteric Tract (LIFT) procedure, or endorectal advancement flap (ERAF). These surgical strategies work best when associated with anti-TNF or immunomodulation and when mild to moderate proctitis is present. More aggressive interventions include diversion of the fecal stream with loop ileostomy and proctectomy; Mesenchymal stem cells have emerged as possible effective treatment and long term results have been demonstrated by randomized clinical trial.
During the past decades, human diet has evolved towards higher intakes of processed and ultraprocessed food. We have investigated the association between these food groups and the risk of inflammatory bowel disease (IBD) in the European Prospective Investigation into Cancer and Nutrition.
Methods413 590 participants from 8 European countries were included. Dietary data were collected at baseline from validated food frequency questionnaires. All EPIC food items were expressed as g/day and categorized according to the NOVA classification: “group 1—unprocessed or minimally processed foods”; “group 2—processed culinary ingredients”; “group 3—processed foods”; and “group 4—ultra-processed foods (UPFs)”. Because the EPIC dietary questionnaires were conceived before the NOVA classification, the food items were retrospectively categorized into this classification. Some food items collected in the EPIC questionnaires were difficult to categorize into group 3 or 4. We therefore merged them into a single category. We tested three scenarios for the estimation of the dietary content of this merged category: lower, middle and upper contents of groups 3 + 4. The association between the proportion of each NOVA group in the diet and IBD were estimated using Cox proportional hazard models to obtain Hazards Ratios (HRs) and 95% confidence intervals. We adjusted the HR for smoking status, educational level, physical activity, BMI, alcohol consumption and energy intake and stratified by centre, age at baseline (1-y interval), and sex.
ResultsAfter a mean follow-up of 16 years, 179 Crohn's disease (CD) and 431 ulcerative colitis (UC) cases were identified. NOVA group 1 was negatively associated with CD risk (adjusted HR for the fourth vs. the first quartile = 0.58; 95% CI = 0.35-0.95; P-trend = 0.03). Within group 1, fruit intake was the only food item to be negatively associated with the risk of IBD (adjusted HR for the fourth vs. the first quartile = 0.42; 95% CI = 0.24-0.73; P-trend = 0.003). We found numerical associations between group 3+4 and the risk of CD with the middle or upper scenarios (adjusted HRs for the fourth vs. the first quartile = 1.50; 95% CI = 0.92-2.46; P-trend = 0.12 and 1.41; 95% CI = 0.88-2.27; P-trend = 0.09 respectively). There was no association between any category of the NOVA classification and UC risk.
ConclusionIn the EPIC cohort, consumption of non-processed food was associated with a lower risk of CD while consumptions of processed or ultra-processed food were numerically associated with increased risk of CD. We found no association with UC.
1) To use IBD prognostic factors to improve management decisions
2) To review why intervene early if poor prognosis
3) To understand the challenges in biomarkers development
4) To learn which promisig biomarkers have been validated for clinical implementation or are undergoing validation
Psychological difficulties are common in people with IBD. In this session I will present the biopsychosocial model of health and discuss how it is involved in the development, maintenance and treatment of IBD. I will discuss how the MDT can support with psychosocial components and why it’s important and briefly discuss the evidence around psychological therapy in IBD.
Educational objectives:
1. To understand the impact of IBD on individual's life e.g. education, employment, relationship, body image, self-esteem
2. To understand the impact of IBD on psychosocial well-being of the individual living with IBD e.g. stress, anxiety, depression, fatigue, food related quality of life, sexuality
3. To review the evidence on psychological well-being of people living with IBD
4. To have an overview of the assessment tools and the support systems available to patients
Eductational objectives:
To give an overview of pulmonary dysfunction / diseases which can occur in IBD patients.
Summary:
Subclinical pulmonary dysfunction occurs in up to 60% of IBD patients. The exact mechanism is unknown, but it has been suggested that the bowel inflammation may shift from the bowel to the lung perhaps because they are embryologically related and immunologically connected.
Respiratory diseases in IBD patients may fall under 4 categories:
1) Tracheobronchial involvement: (tracheo)bronchitis, bronchiectasis, and bronchiolitis; fistulas in Crohn's disease
2) Interstitial lung disease with diverse radiological presentations as nodules, cavities, interstitial pneumonitis
3) Pleuritis, pneumothorax, pulmonary embolism
4) Drug-related pathology: as reported for 5-ASA agents, azathioprine and methotrexate. The widespread use of anti-TNF agents has lead to increased reports of pulmonary infections (tuberculosis and others), and possibly metastatic tumors. Diffuse alveolar damage after infliximab infusion has also been described.
Th17 cells and their main secreting cytokine interleukin-17A (IL-17) are considered as the main pathogenic factors in inflammatory bowel diseases (IBDs). However, anti-IL-17 neutralizing antibodies, a theoretically curative medication for IBDs, paradoxically aggravated intestinal inflammation. The mechanisms by which it mediates the protective and pathologic effects of IL-17 remain unclear in the intestinal epithelium.
MethodsThe intestinal epithelial responses induced by IL-17 was evaluated using the human small intestinal organoid (enteroid) model.
ResultsOrganoid-forming efficiency, cell viability and proliferation of enteroids were decreased in proportion to the concentration of IL-17, which did not differ between the enteroids derived from controls and patients with Crohn’s disease. Bulk RNA-sequencing revealed the enrichment of secretion signaling in IL17-treated enteroids. Among its components, PIGR was up-regulated significantly as the concentration of IL-17 increased, resulting in IgA transcytosis and protective role against pathogens. The IL-17-induced cytotoxicity was predominantly mediated by pyroptosis with activation of CASP1 and cleavage of GSDMD. Single-cell RNA- sequencing identified pyroptosis occurred actively in intestinal stem cells (ISCs) and enterocytes. Anti-IL-17 antibody, izekizumab, completely restored IL-17-induced cytotoxicity, but suppressed mucin secretion and IgA transcytosis. CASP1 inhibitor, Ac-YVAD-cmk, restores cytotoxicity induced by IL-17, without impairing its beneficial effects.
ConclusionIL-17 induces pyroptosis of ISCs and enterocytes, as well as mucin secretion and PIGR-induced IgA transcytosis. Paradoxical gastrointestinal effects of IL-17 neutralizing antibodies may be associated with inhibition of mucin secretion and IgA transcytosis. The inhibition of pyroptosis using the CASP1 inhibitor prevents the cytotoxicity induced by IL-17 without compromising its beneficial effects.
Diagnosis and monitoring of Crohn’s disease (CD) incorporates laboratory parameters and both endoscopic and radiological assessments. Cross-sectional imaging (computed tomography and magnetic resonance enterography [CTE/MRE]), has shown high diagnostic accuracy for detecting small bowel and colonic CD, and provides complementary findings to ileocolonoscopy. Quantitative measurements wall thickness, contrast enhancement, T2 hyperintensity, and direct identification of ulcerative lesions have demonstrated correlation with ileoscopic and histological findings of inflammation. Wall thickening and on MRE correlate with histological findings of inflammation. Following medical treatment, radiological remission [or transmural normalization] by MRE or CTE parallels endoscopic healing with an estimated accuracy of 90%. Additional studies have also shown that the radiological response of inflamed bowel segments to medical therapy is associated with improved long-term outcomes, including reductions in future hospitalization rates or requirement for surgery. Furthermore, cross-sectional abnormalities may exist even in the presence of normal endoscopic examination of the terminal ileum, and even in the absence of histological findings of active inflammation. In these circumstances, patients with normal endoscopy and histology but with persistent cross-sectional imaging alterations face a worse prognosis in terms of relapse, surgery requirements and hospitalizations. Therefore, cross-sectional imaging should be integrated in the definition of complete remission in association with endoscopy and histology.
In a different setting, CT and MRE has demonstrated a high diagnostic accuracy to detect strictures and penetrating complications such as fistulas and abscesses and have a high impact on patient management. Beyond the standard definition of stricture by CTE or MRE, another relevant aspect is the identification and quantification of fibrosis by imaging biomarkers. The proportion of patients developing intestinal strictures increases over the years after a diagnosis of CD and represents the main cause of damage progression and surgery in that group of patients. Different strategies based on MRE have been studied with the aim to detect and quantify the degree of fibrosis deposition, including diffusion-weighted imaging (DWI), magnetization transfer and delayed enhancement. However, recent data from a multicentric study suggests that only DWI shows a good correlation with the fibrosis in the bowel but also with inflammation. Thus, the contribution of additional imaging biomarkers such as the amount of creeping fat or modern stiffness quantification by MR elastography deserves further attention.
Ozanimod, a sphingosine 1-phosphate (S1P) receptor modulator selectively targeting S1P1 and S1P5, is approved in the US for the treatment of moderately to severely active ulcerative colitis (UC). In the pivotal phase 3 True North randomised controlled trial in moderate-to-severe UC, significantly more patients (pts) achieved clinical response and remission with ozanimod vs placebo (PBO) at week (wk) 10 of the induction period. Here, we report the rapidity of ozanimod-induced symptomatic response and remission in pts from True North (NCT02435992).
MethodsIn True North, pts were randomised to once-daily ozanimod 0.92 mg (equivalent to ozanimod HCl 1 mg) or PBO (Cohort 1) or received open-label ozanimod (Cohort 2) during induction. This analysis evaluated symptomatic response (defined as ≥1 point and ≥30% decrease from baseline in adapted partial Mayo score and ≥1 point decrease from baseline in rectal bleeding score [RBS] or absolute RBS ≤1) and symptomatic remission (defined as RBS of 0 and stool frequency score [SFS] ≤1 point and ≥1 point decrease from baseline at each study visit from wk 2 through 10.
ResultsDuring induction, 645 pts were randomised to ozanimod (n=429) or PBO (n=216) in Cohort 1, and 367 pts received open-label ozanimod in Cohort 2. Baseline demographics and clinical characteristics were well balanced across groups. Differences in symptomatic response were observed between ozanimod and PBO recipients in Cohort 1 as early as 2 wk after ozanimod initiation (1 wk post-titration) for the overall population (36.1% vs 26.4%; difference: 9.6% [95% CI, 2.1–17.0]; Figure 1) and tumour necrosis factor inhibitor (TNFi)-naïve pts (38.5%, n=301 vs 29.1%, n=151; difference: 9.4% [95% CI, 0.2–18.5]), and as early as 4 wk for TNFi-exposed pts (42.2%, n=128 vs 27.7%, n=65; difference: 15.8% [95% CI, 1.8–29.8]). Differences in symptomatic remission were observed between ozanimod and PBO recipients in Cohort 1 as early as 5 wk after ozanimod initiation (4 wk post-titration) for the overall population (26.3% vs 16.7%; difference: 8.6% [95% CI, 1.8–15.4] Figure 2), as early as 4 wk for TNFi-naïve pts (27.2% vs 17.9%; difference: 9.4% [95% CI, 1.5–17.4]), and as early as 8 wk for TNFi-exposed pts (22.7% vs 12.3%; difference: 11.7% [95% CI, 1.3–22.1]). Rates of symptomatic response and remission in pts receiving open-label ozanimod (Cohort 2) were similar to those in pts receiving randomised ozanimod (Cohort 1).
ConclusionIn the overall population, ozanimod was associated with higher rates of symptomatic response and remission vs PBO as early as 2 and 5 wk, respectively, after treatment initiation. Both clinical endpoints were more rapidly achieved in TNFi-naïve vs TNFi-exposed pts.

Subcutaneous (SC) formulation of vedolizumab (VDZ) is available for Crohn’s disease (CD) and ulcerative colitis (UC). We assessed the efficacy, safety, and pharmacokinetic (PK) profiles of patients with inflammatory bowel diseases (IBD) who switched from intravenous (IV) to SC VDZ treatment in two prospective, real world cohorts.
The primary cohort is an ongoing open-label, real life, prospective single centre cohort study. As a validation cohort, we used the Initiative on Crohn and Colitis (ICC) registry, a prospective, observational, nationwide registry including patients switching from IV to SC VDZ. In both cohorts, patients receiving IV VDZ maintenance for >4 months were offered to switch treatment to SC VDZ, 108 mg every 2 weeks. In the primary cohort, assessment of clinical, biochemical and PK parameters took place at baseline, at approximately 10 weeks following the switch and at the physician’s discretion thereafter. In the ICC cohort, follow up visits were at week 12 and 24. The primary endpoint was the proportion of patients discontinuing SC VDZ at week 24.
ResultsIn total, 78 (50 CD (64%) and 28 UC (36%)) and 54 patients (29 CD (54%) and 25 UC (46%)) were included in the primary and ICC cohort respectively (table 1). During follow up, 8 (10.3%) of the primary cohort and 6 (11.1%) patients of the ICC cohort stopped VDZ SC during follow-up time till week 24, after a median treatment duration of 18 (IQR=5-19) and 10 (IQR=7-15) weeks, respectively. Treatment withdrawal was most often caused by adverse events (AE), in total for 8 out of 132 patients (6%) (table 2). Four patients had loss of response to SC VDZ. Three of these patients had biochemical disease activity at initiation of SC therapy. Reported AEs included headache and injection related reactions. The median VDZ concentration increased from 11 ug/mL (IQR=9.4-20) to 28 ug/mL (IQR=24.3-31.2, p<0.0001) and from 20 ug/mL (14.3-26.3) to 34.6 ug/mL (26.8-42.9) (p<0.01), between baseline and visit 1 in the primary and validation cohort, respectively (figure 1).

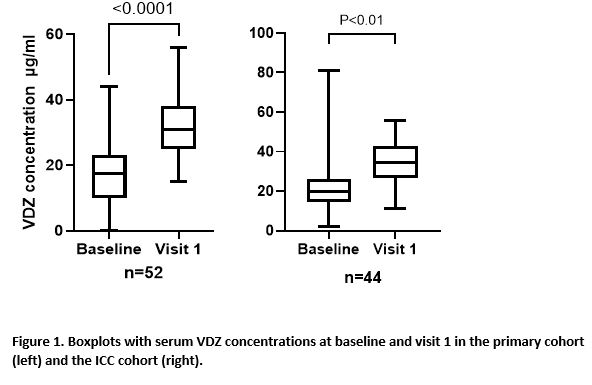

The present abstract reports real world experience of switching IV to SC VDZ maintenance treatment in IBD patients in two observational Dutch cohorts. VDZ concentrations were significantly higher after the switch to SC VDZ. A switch from IV to SC VDZ appears to be effective and safe. However, a proportion of patients switched back to IV VDZ due to injection related AEs.
Several therapeutic options are now available in ulcerative colitis after anti-TNF failure, but no data compared hitherto tofacitinib and vedolizumab.
We compared the effectiveness of tofacitinib and vedolizumab in UC patients with prior exposure ≥ 1 anti-TNF.
MethodsIn this multicentre retrospective study, we consecutively included all adult UC patients with partial Mayo score > 2, with ≥ 1 prior anti-TNF agent and started either tofacitinib (10mg b.i.d ± decreased to 5 mg b.i.d from week 8 (W8)) or vedolizumab (300 mg IV at W0-W2-W6 -W14 [± additional W10]) between January 2019 and June 2021.
The primary endpoint was corticosteroid-free clinical remission or CFREM (partial Mayo score ≤ 2) at W16. Secondary endpoints were endoscopic improvement (CFREM + endoscopic Mayo score ≤ 1) and mucosal healing (CFREM + endoscopic and histological remission defined as Nancy index ≤ 1).
All the comparisons were performed using propensity score analyses (inverse probability of treatment weighting) adjusted on gender, smoking, UC duration and extent, number of prior biologics or prior primary failure to biologics, concomitant 5-ASA, steroids or immunosuppressive agents, and disease severity.
ResultsOverall, 400 patients will be included. Among the 200 first patients, 87 and 112 received tofacitinib and vedolizumab, respectively (one missing patient). Except for more pancolitis (54.0% vs 38.4%, p=0.028), less immunosuppressive therapies (4.6% vs 27.7%, p < 0.001), and higher rate of prior exposure ≥ 2 biologics (87.4% vs 37.5%, p < 0.001) in tofacitinib arm, baseline characteristics were similar across the two groups including concomitant 5-ASA (10.3% vs 18.8%) and steroids (23.0% vs 31.2%). Vedolizumab infusion at W10 was performed in 34.3% while 42.5% received tofacitinib 10 mg b.i.d until W16.
CFREM was achieved in 54.2% and 42.5% in tofacitinib and vedolizumab, respectively p=0.089). The rate of CFREM at W16 was 57.4% vs 51.1% (p=0.77) after one biologic, 55.4% vs 41.8% (p=0.61) after 2 biologics, 56.9% vs 6.3% (p=0.007) after at least 3 biologics, and 59.0% vs 33.3% (p=0.17) in the subgroup with partial Mayo score ≥ 6, in tofacitinib and vedolizumab groups, respectively.
Tofacitinib was more effective than vedolizumab to achieve CFREM at W16 in patients with primary failure to at least biologic (71.6% vs 30.8%, p=0.049).
Among 177 patients, endoscopic improvement was higher in patients treated with tofacitinib (33.6 % vs 7.1%, p=0.048). Mucosal healing was observed in 6.4% vs 3.8% in tofacitinib and vedolizumab arms, respectively (p=0.27).
ConclusionTofacitinib and vedolizumab are effective after failure to anti-TNF agents. Tofacitinib seems to be more effective in case of primary failure to biologics and multiple therapeutic failure.
1. To understand why relationships, intimacy and sexuality are important to those living with IBD
2. To discuss the sexual well-being concept
3. To review the current evidence on sexual well-being concerns from patient perspective
4. To review the current evidence of meeting sexual well-being and family planning care needs
5. To have an overview of optimal strategies to provide holistic care incorporating aspects of sexual well-being
1. To introduce changing targets for remission
2. To discuss impact of `deep` remission in UC and CD
3. Describe challenges to meeting new targets
In asymptomatic patients with inflammatory bowel disease, monitoring involves repeated testing aimed at early recognition of a disease flare. The ultimate goal is to restore disease remission as early as possible and to prevent disease progression. Commonly used biomarkers to assess IBD activity include C-reactive protein (CRP) and fecal calprotectin (FC).
Classically, these diagnostic tests are performed in hospital laboratories where quality-control procedures apply. Recently, FC home testing has become available, allowing patients to measure IBD activity themselves. To establish a remote monitoring service that provides reliable results for correct clinical decision-making, a number of essential quality criteria must be met. In this presentation I outline the principles to good remote monitoring and illustrate those principles with an example from practice.
Educational objectives:
1. To discuss 10 things to know before starting a remote monitoring service
2. To understand that setting up a remote FC monitoring service requires cooperation between health professionals, laboratory and medical IT specialists.
3. To emphasise that measuring biomarkers without accepting the consequences of the result is a waste of money.
Educational Objectives:
1. Definition of remote monitoring
2. Patient´s view: what would I expect
3. Early relevant telemedicine IBD papers
4. Role of calprotectin (point of care testing, POCT)
5. Does POCT affect medical therapy?
6. Lipocalin 2: a new fecal stool marker in IBD?
7. Patient´s view: where do I see the future?
8. Remaining challenges
Educational objectives:
1. To understand the process of research application preparation
2. To have an overview of good research application for funding
3. To have an overview of the research funder's requirements
4. To continue the networking in the Senior group of nurses in research
5. To find different kind of collaborations in the group
