1. To provide an overview of some of the nutrition and dietary management options for IBD
2. To highlight the role of the IBD nurse in relation to nutrition and dietary management of IBD
1. To establish a standard terminology for the elaboration of trusted reports in endoscopy, surgery and histopathology
2. To provide i interpretation guidance for dysplasia and cancer, surgical indication and strategies including margins and extent of resection, assessment of disease activity.
3. To standardise the interpretation of biopsies and specimens.
4.To prove useful these recommendations in daily care of IBD patients
Disruption of immune cell trafficking via integrins is a proven and effective mechanism for treating inflammatory bowel disease. When α4β7 integrin is inhibited through pharmacological intervention, immune cells destined for the gut tissue become sequestered in blood circulation and these alterations can be detected through several methods. MORF-057 is a novel, oral, selective, small molecule inhibitor of α4β7 integrin developed for treating IBD. MORF-057 demonstrated favorable tolerability, pharmacokinetic and pharmacodynamic profiles including saturating receptor occupancy and corresponding evidence for proof of biology based on effects on circulating cells during a Phase 1 clinical trial in healthy volunteers (Ray, ECCO 2021). Here we demonstrate an exposure:response relationship of α4β7 related biomarkers examined among MORF-057 treated non-human primates (NHPs) as a means for pre-clinical testing of inhibitors of this pathway.
MethodsSingle-cell RNA sequencing (scRNAseq) was performed on NHP CD45+ blood cells to determine baseline populations potentially impacted through exposure to MORF-057. 40 Naïve cynomolgus monkeys were enrolled over 5 separate studies and dosed orally BID with MORF-057 over 2-7 days using several dose levels to examine biomarker dynamics over a wide range of exposures. Peripheral blood was sampled at various timepoints (n= 125) and assayed using: mass spectroscopy, flow cytometry (FACS), and mRNA quantification to determine MORF-057 exposure levels, on-target receptor occupancy (RO), immune cell subset changes, and CCR9 mRNA levels.
ResultsMORF-057 Ctrough ranged from 3.3-429 ng/ml. In samples tested for RO, MORF-057 achieved >95% saturation of α4β7 even with the lowest Ctrough of 4.5 ng/ml. MORF-057 treatment led to significantly sustained increases in circulating β7high CD4+ T memory cells detectable as early as 24h post-exposure. A maximal effect where β7high cells accounted for approximately 60% of the T memory population was achieved at saturating receptor occupancy (Fig., left). CCR9 mRNA values demonstrated increases upon drug exposure (Fig., right).
In NHP, acute changes in circulating β7high T memory cells was a sensitive biomarker demonstrating a dose-dependent response to MORF-057 exposure. CCR9 mRNA levels also showed similar exposure related changes reflecting its expression on a subset of β7high cell types. Analysis of scRNAseq shows expression of α4β7 on other cell types beyond T memory cells including: NK, NKT, B cells, plasmablasts, monocytes, and eosinophils. Pharmacodynamic changes in NHP are consistent with human Phase 1 data in healthy volunteers.
Limited data are available on the use of Vedolizumab (VDZ) in paediatric Crohn’s Disease (CD) and Ulcerative Colitis (UC). We evaluated the effectiveness and safety of VDZ to induce remission at week 14 in the prospective, multicenter VEDOKIDS study.
We enrolled children (age 0-18 years) with CD or UC commenced on VDZ with a standardized dosing of 177mg/BSA up to 300mg at 0, 2, 6 and q8 weeks thereafter. Non-responders had their dose escalated to q4wks at the discretion of the local physician. Explicit demographic, clinical and safety data were prospectively recorded via REDcap. Clinical remission was defined as steroid- and EEN-free remission (i.e. wPCDAI<12.5 or PUCAI<10) without the need for new medications. Complete remission was defined as clinical remission with normal CRP and ESR. Predictors of response were explored by Logistic regression.
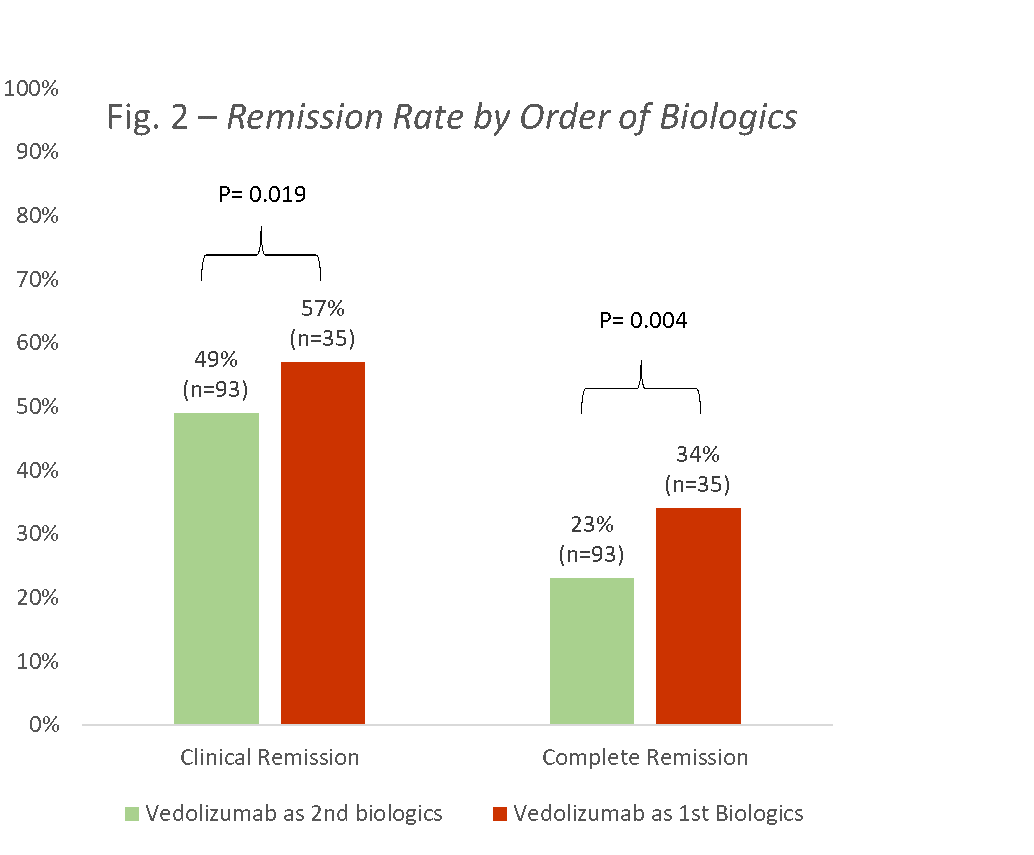
Results128 children were enrolled, 60 (47%) with CD, and 68 (53%) with UC (58 (45%) males, mean age 13.8±3.6, 93 (73%) failed previous anti-TNF, median disease duration 2.3 years (IQR 0.9-4.7)). Using the ITT principle, clinical and complete remission rates for CD at week 14 were 30% and 20%, respectively, and for UC 50% and 38%, respectively (Fig 1). Clinical remission rates of those receiving VDZ as first line biologics versus second line were 57% and 34%, respectively (p=0.019; Fig 2); the corresponding complete remission rates were 49% and 23% (p=0.004).
In the UC group, disease activity at baseline measured by the PUCAI predicted clinical remission at week 14 (OR=0.95, 95%CI 0.93-0.98; median baseline PUCAI 15 (IQR 0-30) in those achieving remission and 45 (20-55) in those who did not; p=0.002). ESR (OR=0.94, 95%CI 0.89-0.98; p=0.009) and a trend towards extensive disease (L3 vs. L1 and L2; OR 0.14, 95%CI 0.18-1.036, p=0.054) predicted clinical remission in CD.
During the 14 weeks, 113 adverse events (AE) were recorded in 58 children: 28 AEs were possibly related to VDZ, all of which were mild-moderate and only 3 (11%) led to discontinuation of VDZ (leukocytoclastic vasculitis, myalgia and dyspnea). There were 18 serious AEs, only one was graded as possibly related to VDZ (headache). There were 18 non-serious cases (19%) of upper respiratory infections (pharyngitis, tonsilitis, parotitis, and otitis media) and one Campylobacter jejuni which was graded as serious.

In this prospective multicenter study, VDZ was safe and effective for inducing remission in a refractory cohort of paediatric IBD, more so in UC. Disease severity and extent at baseline may predict clinical response.
MDT discussion / panel discussion Q&A
IBD Multidisciplinary Team (nurses, dietitians, pharmacists, psychologists, etc.)
1. To review the clinical key predictors of poor outcome in IBD
2. To understand the progress made in predicting the future for a given IBD patient
3. To learn how to communicate prognostic factors to to the patients
Genetics plays a key role in the pathogenesis of inflammatory bowel disease (IBD). With the expanding use of next-generation sequencing, >70 different monogenic disorders associated with IBD have been identified, and most of them present in the first years of life. Recently, several patients with severe IBD were identified to harbor pathogenic mutations in Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) gene, which regulates necroptosis, a necrotic cell death mechanism. We present the clinical features, genetic analysis and immune work-up of three patients with infantile-onset IBD resulting from novel RIPK1 mutations.
MethodsWhole exome sequencing was performed in three patients with severe infantile-onset IBD, along with sanger sequencing for conformation. Mass cytometry time of flight was conducted for in-depth immunophenotyping, including cytokine secretion analysis following lipopolysaccharide (LPS) or Phorbol myristate acetate with ionomycin (PMA-I), on one of the patient’s peripheral blood mononuclear cells, and compared to control subjects and patients with Crohn’s disease.
ResultsAll patients, born to consanguineous Muslim families, presented with severe colitis and multiple perianal fistulas in the first months of life, without severe or atypical infections. One of the patients had a partial response to high doses of inlfliximab and azathioprine, while another one failed to respond to adaliumab and later to low dose anakinra, an IL-1 receptor antagonist. Genetic studies identified novel and pathogenic genetic variants in the RIPK1 gene in all patients, that were confirmed by Sanger sequencing. Using mass cytometry time of flight unbiased clustering analysis, we identified peripheral immune dysregulation in one of these patients, characterized by an increase in IFNγ CD8+ T cells along with a decrease in monocytes, dendritic cells and B cells. Moreover, RIPK1-deficient patient’s immune cells exhibited decreased IL-6 production in response to LPS across multiple cell types including T cells B cells and innate immune cells.
ConclusionMutations in RIPK1 should be considered in very young patients presenting with colitis and perianal fistulas. Given RIPK1’s role in both immune cells (and specifically in inflammasome activation), and epithelial cells, it is unclear whether immunosuppressive medications (including IL1 blockade) as well as allogeneic hematopoietic stem cell transplantation can suppress or cure the hyper-inflammatory response in these patients. Additional studies in humans are required to better define the role of RIPK1 in regulating intestinal immune responses, and how treatment can be optimized for patients with RIPK1 deficiency.
The presentation will focus on how to monitor patients' symptoms due to IBD, how to use the main scores and how to use the patients' reported outcomes in clinical practice.
Educational objectives:
1) To understand how important are symptoms in the monitoring of IBD patients
2) To make on overview on the main clinical scores
3) To present the recent evidence on patients' reported outcomes
Risankizumab (RZB), a selective interleukin-23 inhibitor, demonstrated clinically meaningful improvements in endoscopic outcomes in patients with moderate to severe Crohn’s disease (CD) during two phase 3 induction trials (ADVANCE and MOTIVATE) and the maintenance study (FORTIFY). Here, we compared the efficacy of RZB in inducing and maintaining improvements in endoscopic outcomes in patients with CD who demonstrated intolerance and/or inadequate response (IR) to biologic therapies (with prior bio-failure) versus those who demonstrated IR to conventional therapies only (without prior bio-failure).
MethodsData included in this subgroup analysis included pooled data from patients randomized to receive intravenous (IV) RZB 600mg (N=527) or placebo (PBO) IV (N=362) every 4 weeks (wks) for 12wks during induction (ADVANCE+MOTIVATE), and data from patients receiving subcutaneous (SC) RZB 360mg (N=141) or withdrawn from RZB IV to receive PBO SC (withdrawal [PBO SC], N=164) every 8wks for 52-wks during maintenance. At Wks 12 and 52, endoscopic response, endoscopic remission, ulcer-free endoscopy (absence of ulceration), and deep remission (Wk52 only) were evaluated both in the overall population and in subpopulations of patients with and without prior bio-failure. (Endpointsare defined in Table footnotes). Safety was assessed throughout the studies.
ResultsApproximately three-quarters of randomized patients included in this subgroup analysis had prior bio-failure (ADVANCE+MOTIVATE: 75.4%; FORTIFY: 73.8%). Higher rates of endoscopic response, endoscopic remission, and ulcer-free endoscopy were observed at Wk12 among patients receiving induction with RZB IV versus PBO IV. Subgroup analysis demonstrated treatment effects with risankizumab in patient subpopulations with and without prior bio-failure, with greater adjusted differences versus PBO in patients without prior bio-failure (Figure). At Wk52, endoscopic response, endoscopic remission, ulcer-free endoscopy, and deep remission rates favored RZB SC compared to withdrawal (PBO SC). Again, treatment effects were observed in patients with and without prior bio-failure, with greater adjusted differences versus withdrawal (PBO SC) in patients without prior bio-failure. RZB maintenance treatment was well-tolerated and no new safety signals were observed. The safety profile of RZB has been reported previously.1,2
Induction and maintenance therapy with risankizumab achieved higher rates for endoscopic endpoints in patients with moderate to severe Crohn’s disease versus placebo, regardless of prior bio-failure status. However, numerically higher efficacy rates were observed in patients without prior bio-failure.
1 D’Haens, G. et al. in DDW 2021 2 Ferrante, M. et al. in UEGW 2021
Perianal fistulas are a common complication of Crohn’s disease (CD) affecting approximately 25% of patients, often predicting a more complicated disease course. Dysregulated immune responses and epithelial-to-mesenchymal transition (EMT) are hypothesised to contribute to fistulizing disease; however, they have been poorly studied. In this study, we investigated the immune phenotype of patients with perianal fistulizing disease and its relationship with tissue remodelling.
MethodsImmune cells were isolated from fistula curettage samples (n=31) and paired peripheral blood from patients with perianal Crohn’s (pCD) or idiopathic fistulizing disease. Multiparameter flow cytometry was performed to evaluate lymphocyte populations including invariant natural killer T-cells (iNKTs), gamma-delta (γδ) T-cells, CD161+ mucosal-associated invariant T-cells (MAIT), CD4+ T-cells and CD8+ T-cells. Gene expression profiling of fistula (Crohn’s n=11, idiopathic n=11) and rectal tissue (Crohn’s n=9, idiopathic n=9) was performed by RNA-sequencing. Cellular deconvolution of transcriptomic data using CIBERSORTx was performed to define the cellular phenotype of perianal fistulas. Cytokine treated intestinal epithelial organoids were used to probe the impact of selective cytokines on disease relevant pathways in perianal fistulas, using gene-set enrichment analysis (GSEA).
ResultsPerianal fistulas were characterised by expansion of iNKTs and CD4+ T-cells when compared to peripheral blood. Deeper analysis of the phenotype of these populations revealed enrichment of CD8- CD4- CD161+ iNKTs producing interleukin-22 (IL22), and CD4+ CD161+ T-cells producing interleukin-13 (IL13) and IL22. Surprisingly, pCD and idiopathic fistulas displayed similar immunophenotypes. Fistulas exhibited distinct transcriptional profiles to rectal tissue, although the phenotype of pCD and idiopathic fistulas appeared similar. Pathways related to the extracellular matrix (ECM) and EMT were more activated in fistulas compared to rectum. Gene-set enrichment analysis and cellular deconvolution identified an increase in the abundance of iNKTs, activated memory CD4+ T-cells, activated NK cells and neutrophils in fistula versus rectal tissue. IL13, IL22 and TNFα responsive transcripts were enriched in fistula tissue, and in the case of IL22, was shown to regulate key matrisome components.
ConclusionDespite the proven efficacy of vedolizumab (VDZ), only 29% and 36% of the Crohn’s disease (CD) patients present corticosteroid-free clinical- and endoscopic remission, respectively. Therefore, predictive biomarkers for treatment success would be of extreme value. Previous studies have identified aberrant DNA methylation associated with CD-specific phenotypes, suggesting that the methylome may be useful for classification and prediction of VDZ treatment response. Here, we sought to identify such DNA methylation biomarkers that can predict clinical- and endoscopic response to VDZ in CD patients.
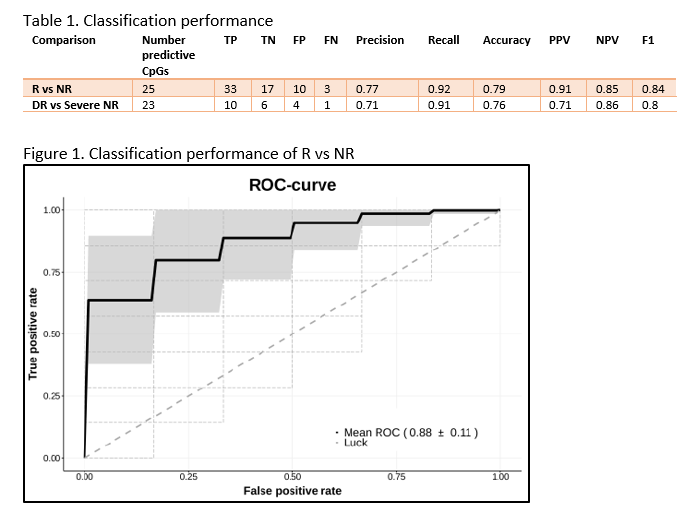
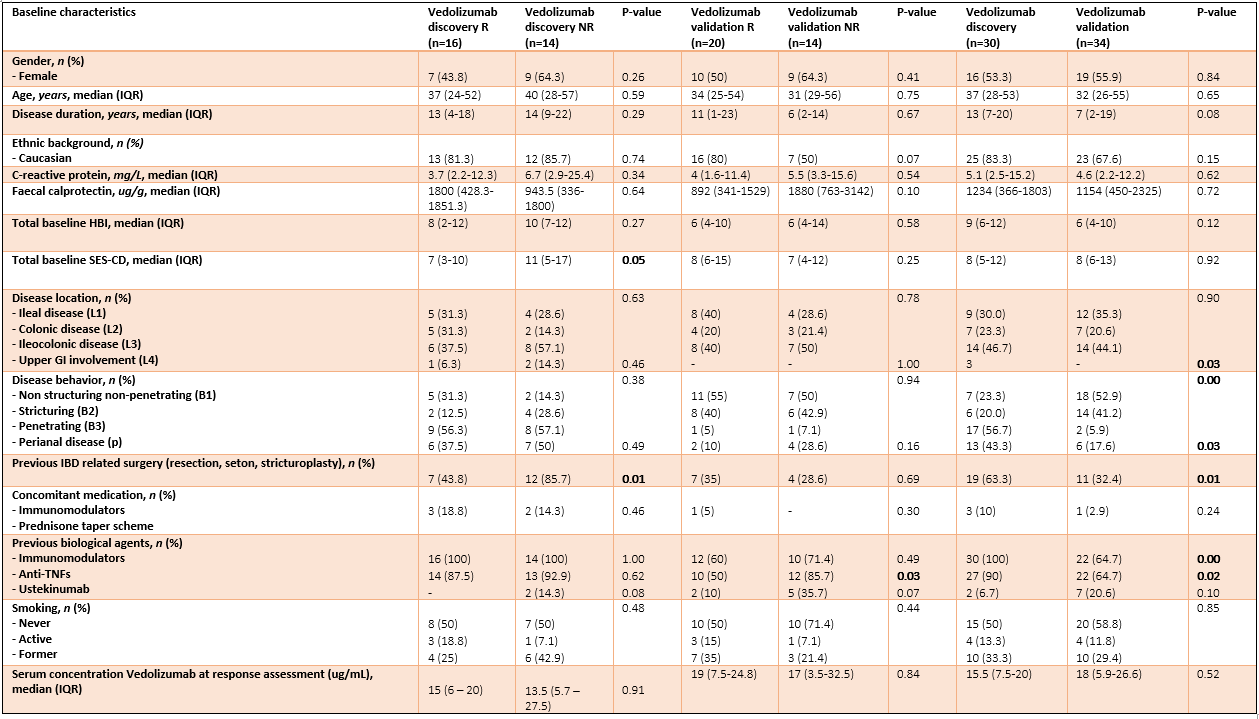
MethodsWe prospectively recruited adult CD patients that initiated VDZ treatment following a baseline colonoscopy in two cohorts: a discovery and validation cohort. Peripheral blood DNA methylation profiles were measured prior to treatment (T1), and after a median of 22 weeks (T2) using the Illumina Infinium HumanMethylation EPIC BeadChip array. Response (R) was defined as the strict combination of endoscopic- (≥50% reduction in SES-CD score) and steroid-free clinical response (≥3 point drop in HBI and HBI ≤4 AND no systemic steroids) and/or biochemical response (≥50% reduction in C-reactive protein (CRP) and fecal calprotectin or a CRP ≤5 g/mL and fecal calprotectin ≤250 µg/g). Twenty-one patients had deep remission (DR), defined as a combined endoscopic- (SES-CD≤2) and steroid-free clinical remission (HBI ≤4, no systemic steroids). Biomarker identification and classification analyses were performed using stability selection gradient boosting.
ResultsIn total, 64 CD patients were enrolled (discovery 16R/14NR and validation 20R/14NR). Both cohorts were comparable for age, sex and smoking status. Forty-nine (77%) patients had previously failed an anti-TNF agent. All patients had measurable serum vedolizumab concentration at T2 (median 14.5 (6.9 – 21.3) µg/mL). Through classification analysis at T1, we were capable of discriminating R from NR with high predictive performance (25 CpGs, AUC 0.88, F1-score of 0.84, PPV of 0.91 and NPV of 0.85). When analysing the methylome of patients in deep remission, we identified 23 CpGs with high predictive performance upon independent validation (F1-score 0.80, PPV of 0.71 and NPV of 0.86). Investigating the CpGs of interest implicated genes involved in endothelial cell-cell adhesion and integrin dependent T-cell homing, corroborating VDZ’s mode of action.
We demonstrate two novel 25- and 23-feature panels of epigenetic biomarkers that accurately predict response or deep remission to vedolizumab respectively. Similar analyses on infliximab, adalimumab and ustekinumab are currently ongoing as part of the EPIC-CD study.
Inflammatory bowel disease (IBD) exhibits heterogeneity at genetic, phenotypic, and therapeutic levels [1]. Although several studies have investigated genetic effects on IBD subtypes and drug adverse events [2,3], few have comprehensively explored the phenotypic and genetic determinants of IBD drug efficacy in a sufficiently powered cohort.
MethodsWe used data from 32,199 patients in the UK IBD BioResource to investigate the effects of clinical phenotypes and demographics on drug efficacy and combined this with genome-wide genetic data for a subset of 11,536 individuals (Table 1). Drug efficacy was defined using a combination of clinician reported efficacy and persistence on drug without treatment escalation. Anti-TNF, thiopurine, steroids, and mesalazine were explored. We estimated phenotypic effects on drug efficacy using multivariable logistic regression and the genetic effects by genome-wide logistic regression. To explore whether drug efficacy and IBD disease susceptibility share a genetic basis, we estimated the proportion of variance in drug efficacy explained by known IBD risk variants [4]. Associations with Bonferroni corrected P-values < 0.05 were defined as statistically significant in phenotypic analyses and a genome-wide significance threshold of P=5x10-8 was adopted for genetic analyses.
ResultsDrug efficacy was generally lower in patients with Crohn’s disease (CD) compared to those with other subtypes (OR ranges from 0.40 to 0.79), but anti-TNF showed a higher efficacy rate (OR = 1.21) in CD patients. Increasing age at diagnosis was associated with evidence of increased efficacy of thiopurine and mesalazine (Table 2). We found evidence of a genetic contribution to variation in drug efficacy for most drugs studied. However, known IBD risk variants showed little contribution (Figure 1). Using genome-wide association testing, we identified three loci showing a significant association with drug efficacy; two were related to steroid response and one to thiopurines (Table 3). None of these was an IBD disease susceptibility locus (P > 0.05).
ConclusionUsing a large, well-characterised cohort we found both genetic and phenotypic determinants of drug efficacy. Three loci were reported to be associated with drug efficacy in the first phase of the genetic analysis; at least 4,200 extra genotyped samples will be included before the ECCO meeting, thus increasing the power to detect additional loci. Our results suggest the genetic causes of drug efficacy and disease susceptibility are largely independent. These findings may provide opportunities for exploring the biology of drug efficacy and improving medication prioritization in IBD patients.
Reference
1. PMID: 16819502
2. PMID: 26490195
3. PMID: 30806694
4. PMID: 28067908



Educational Objectives:
1. Definition of point of care testing (POCT)
2. Patient´s view: why would I prefer POCT?
3. Fecal alprotectin (FC) – prototypic fecal marker of inflammation
4. Clinical studies assessing FC in POCT
5. Role of therapeutic drug monitoring
6. Effect of Covid-19 on POCT development
7. Lipocalin-2: a new fecal marker of inflammation
8. Outlook and challenges for POCT
Perianal Crohn's disease (pCD) may present with a variety of lesions that include anal skin tags, anal canal lesions including fissures, ulcers, fistula and abscesses, strictures and cancer. pCD is disabling and aggressive phenotype that negatively impacts on the quality of life of affected patients. Perianal fistulas are common manifestations of pCD, with an incidence of 11 cases per 1000 patient-years. Successful treatment of pCD remains still a struggle for both physicians and patients. Significant advances in the management of pCD have occurred in the last two decades, resulting in the concept of a collaborative multidisciplinary approach using the latest medical therapies combined with modern surgical or endoscopic techniques. The use of antibiotics in the treatmentof pCD has limited evidence and antibiotics are currently used in combination with other therapies to prevent abscess formation and improve the rate of fistula closure. Thiopurines in pCD lacks of prospective trials, and evidence supporting their use should be carefully interpreted. Although anti-TNFs have revolutionized the prognosis for patients with pCD in the modern era, their effectiveness in the long term is limited: over 60% may relapse after one year of treatment and less than one third mantain sustained remission over time. Optimisation of anti-TNF treatment in pCD includes associaton with an antibiotic for the induction of fistula remission, combination with thiopurines to achieve appropriate anti-TNF trough level and a low likelihood of anti-drug antibodies. The use of vedolizumab and ustekinumab in pCD after anti-TNFs failure is mainly supported by one randomized phase IV trial (vedolizumab) and a number of real life reports (vedolizumab and ustekinumab), suggesting that they could be beneficial in patients who have failed to respond to anti-TNFs. Finally, in case of refractory pCD, a number of mesenchymal stem cell (MSC)-based therapies have been reported. In particular the use of darvadstrocel in refractory pCD is supported by phase 3 trial, showing that local treatment with adult allogeneic expanded adipose tissue-derived mesenchymal stem cells may succeed in 50% of treated patients after 6 months.
Learning Objectives:
1. Aspects of risk stratification
2. Utility of drugs that may alter the natural history (reducing rates of surgeries and complications)
3. Timing of intervention
4. Evidence base data comparing different classes
5. Economic consideration/medical economics including the utility of biosimilars
Several potential risk factors for disease recurrence after surgery have been identified, includingage, disease phenotype, and smoking. In the light of the evolution of prevention and management ofpostoperative recurrence, including early immunosuppressive and biologic treatments, theidentification of potential risk factors is pivotal. Despite the clinical significance, few studies haveinvestigated the association between postoperative complications and recurrence in Crohn’s disease(CD) patients after primary ileocolonic resection. A retrospective analysis of consecutive patientsundergoing primary ileocolonic resection for CD at two European referral centers was performed toaddress the effect of postoperative complications on endoscopic and clinical recurrence. Data from262 patients were retrieved from the Institutional databases: 145 patients developed endoscopicrecurrence and 117 patients were recurrence-free. At multivariable analysis, smoking, penetratingphenotype, perianal disease, and postoperative complications were risk factors for endoscopicrecurrence. Postoperative complications and penetrating disease significantly reduce the time toendoscopic recurrence; postoperative complications and penetrating disease significantly shortenthe time to clinical recurrence. In summary, postoperative complications rate was an independentrisk factor for endoscopic recurrence after primary surgery for CD and affected the rate and timingof endoscopic and clinical disease recurrence.
The current novel coronavirus (SARS-CoV-2) pandemic is an ongoing global health crisis, which represents an important challenge for the whole society and mankind. Patients with inflammatory bowel disease (IBD) are treated with immunosuppressive drugs that are usually associated with more severe viral infections. However, the effects of the different therapies on the risk of SARS-CoV-2 infection and Covid-19 severity in IBD patients are still under investigation.
MethodsBetween April 2020 and April 2021, 238 IBD patients (N=145 with Crohn disease and N=93 with Ulcerative colitis) of the North Italy area have been enrolled. Both serum samples (N=238 IBD patients and N=45 healthy donors) and colon biopsies from inflamed and non-inflamed mucosa (N=88 IBD patients N=20 non-IBD control) have been collected. To evaluate the exposure to SARS-CoV-2, both clinical data and seroprevalence of anti-SARS-CoV-2 Ab have been analyzed. Serum samples were analyzed by untargeted metabolomics analysis and the frequency of a serum metabolomics signature associated with protection were evaluated in IBD versus healthy donors. Moreover, gene expression analysis of key proteins for virus entry (ACE2, TMPRSS2, TMPRSS4, ADAM17) were analyzed by qPCR in the gut mucosa biopsies of IBD patients.
ResultsThe seroprevalence of anti-SARS-CoV-2 Ab in our cohort of IBD patients (10/238) indicates an overall lower incidence of COVID-19 in comparison with the general population of Lombardy. Accordingly, we observed that the serum metabolomics signature associated with protection was more frequent in IBD patients treated with anti-TNF (N=50, 70%), than healthy controls (N=45, 50%). Gene expression analysis of the proteins involved in SARS-CoV-2 entry also indicated that IBD patients treated with anti-TNF (N=14) had a lower mucosal level of SARS-CoV-2 receptor ACE2 and its sheddase ADAM17 than non-IBD subjects along with higher expression of the proteases TMPRSS2 and TMPRSS4. Moreover, vedolizumab-treated patients (N=7) showed a significant lower expression of ACE2, TMPRSS2 and TMPRSS4 than controls, whereas ADAM17 levels were similar.
ConclusionOur study indicates that IBD population treated with biologics has an overall lower risk to contract SARS-CoV-2 infection. Future studies to gather the mechanisms underpinning the effects of biologics on the expression of the proteins involved in SARS-CoV-2 viral entry in association with the specific metabolomics signature of viral susceptibility might shed light on potential targets to increase resistance in higher risk subgroups of patients.
The ileal pouch-anal anastomosis (IPAA) is the gold standard to restore intestinal continuity inpatients with ulcerative colitis (UC), familial adenomatous polyposis (FAP), and in selected patientswith Crohn’s disease (CD) undergoing proctocolectomy. IPAA produces reasonable long termresults and is associated with low mortality as well as good patient satisfaction. However, long-term pouch failure may occurs in a minority of cases. Various IPAA-related complications such asanastomotic leakage, pelvic sepsis, fistula, stricture, pouchitis and “crouchitis” are associatedwith pouch failure. Pelvic sepsis is reported to be the most important risk factor for pouch failure.The rate of IPAA-related complications varies widely in the literature and may have increased inthe era of biologics. However, ambiguous definitions for anastomotic complications, differences inpostoperative assessment, and duration of follow-up make a comparison of outcomes followingIPAA challenging. Although re-do pouch surgery is feasible it has been generally associated withworse outcome when compared to primary surgery. For this reason pouch surgery has the right tobe considered as a “once in a life time surgery”. To reduce the risk of pelvic sepsis, the focus hasbeen on optimization of preoperative performance status, staged procedures, fecal diversion, andadequate postoperative management (ie, early detection and pro-active treatment of anastomoticleaks). From a technical point of view, since its introduction in 1978, restorative proctocolectomyhas gone through a progressive evolution including the application of stapled anastomosis,minimally invasive approach and transanal technique. Transanal techniques and single stapledanastomosis have the potential to standardize the length and shape of the rectal cuff and thereliability of the anastomosis respectively with subsequent impact of long term outcomes in termsof function.
The management of inflammatory bowel disease (IBD) requires a personalized approach to manage a heterogeneous group of patients with variable disease courses. Precision care in IBD involves identifying patients at high risk for rapid progression to complications, selecting the most appropriate therapy for a given patient, predicting response to therapy and safety of drugs. Personalized medical decisions may allow specific therapeutic plans to draw on serologic, genetic, and microbial data to optimise treatment outcome.
The HLA-DQ polymorphism is likely to become important for patient stratification. Over 30% of patients with IBD have this polymorphism. Variants in the HLA-DQA1*05 allele are associated with greater likelihood of immunogenicity and this risk can be greatly reduced by use of a concomitant immunomodulator. Patients with high levels of a blood cytokine level, Oncostatin M, are also less likely to respond to anti‑TNF. Integrating multi-omics including faecal metagenomic, serum metabolomic and proteomic profiles can predict differential response to anti-cytokine or anti-integrin therapy. In predicting treatment safety, leukopenia-free survival is improved when genotyping for NUDT15 prior to therapy initiation with subsequent genotype-based dosing in a Japanese cohort.
Patients with a more diverse baseline microbiome and higher microbial diversity showed better response to anti-TNF agents, vedolizumab, and ustekinumab. Fewer mucus-colonising bacteria, a higher abundance of short-chain fatty acid-producing bacteria, and lower abundance of pro-inflammatory bacteria are also associated with a favourable outcome. The microbiome may also play a role in determining which patients can stop treatment once they are in deep remission. In the future, the combination of metabolomic, metataxonomic, or metagenomic profiling can further enhance precision medicine in IBD.
The insufficient effects of current medical IBD therapies have led to the contention that therapeutic approaches can be improved by personalizing care using precision medicine. This process entails an effort to combine clinical patient characteristics and mechanistic drug effects and align them with therapeutic outcomes, thereby providing biomarkers for selecting the appropriate drug for individual patients. Significant challenges for identification of response biomarkers include patient heterogeneity and the complexity of drug response mechanisms.
An analytic approach based on molecular correlation networks may allow to perturbate this complexity and provide necessary insights to employ this strategy.
We used the comparison of disease versus healthy immune landscape and immune system dynamics during therapy, combined with assessment of individual immune responses to approach this complex landscape.
Using this approach, we identified peripheral blood baseline expression of the cytoskeleton RAC/PAC pathway as a response biomarker for infliximab therapy, both in Crohn’s disease and Rheumatoid arthritis patients, thus identifying a patient-specific rather than disease-specific biomarker.
Notably, this pathway was also associated with the TREM adaptor (TYROBP/DAP12) downstream to TREM-1, which we previously found to be predictive for anti-TNF response. When tested in a control cohort of vedolizumab responsive patients, this biomarker was found to be infliximab-specific and responsive to therapy. Pathway expression was predominantly driven by intermediate monocytes.
Furthermore, identification of the RAC/PAC pathway as central to infliximab response, provides an additional potential mechanistic explanation for the documented synergistic effects of anti-TNF thiopurines combination, translating the findings into clinically relevant established observations.
Leveraging this approach may also allow for future discovery of other effective drug combinations and novel therapeutic compounds.