Ulcerative proctitis (UP), defined as a colonic location limited to the rectum, is a poorly investigated condition in children, usually considered as a minor form of Ulcerative Colitis (UC). The aim of the present study was to compare the disease course of paediatric patients affected by UP at diagnosis with the other UC locations.
MethodsThis multicentre retrospective observational study has been carried out starting from the data prospectively registered in the IBD Registry of the Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition (SIGENP). Seventeen IBD referral centres adhering to the registry were included in the study. Patients age 0 to 18 years, who were diagnosed with UC according to the Porto criteria starting from January 1, 2009, to May 1st, 2021 were identified. Only children with a minimum follow-up of 12 months were included in the study. Once enrolled, children were subsequently divided in two groups based on Paris classification: group 1 (E1) and group 2 (E2, E3 and E4).
ResultsEight-hundred-eighty-five children were finally included in the study (median age at diagnosis: 11.2 years, range: 0-18 years; M/F: 434/451), of whom 176 (19.8%) belonging to group 1 and 709 (80.1%) to group 2. The median age at diagnosis was significantly higher in group 1 when compared to group 2 [11.9 (0-18) versus 11 (0-18) years, respectively; (p<0.001)]. At diagnosis, the induction therapy was significantly different with 68 (39.5%) patients of group 1 undergoing steroid therapy versus 505 (71.2%) of group 2 (p<0.001) and 79 (41.9%) of group 1 practising only mesalamine respect to 186 (26.2%) of group 2 (p<0.001). A higher number of children from group 2 started immunosuppressive or biologic therapy as maintenance therapy at diagnosis [Group 1: 11 (6.2%) versus 173 (24.4%), respectively; (p<0.001)]. The median follow-up of our cohort was 4.5 years (range 1-13 years). At the last follow-up, 67/176 (38%) children with UP showed an extension of their disease location without significant difference when compared to group 2 [265 (37.5%); p=0.9], while 81 (45%) children from Group 1 were under immunosuppressive or biologic therapy versus 566 (79.8%) from group 2 (p<0.001). Five children (3%) of Group 1 underwent colectomy during the follow up versus 45 (6.9%) of Group 2 (p=0.06).
ConclusionUP is a frequent location of paediatric onset UC and the risk of endoscopic extension of proctitis is similar to the more extensive forms. A considerable number of patients with UP required immunosuppressive or biologic therapy during the follow-up and no significant difference was observed in terms of surgery. Overall, UP cannot be considered as a minor form of UC.
In recent decades, there has been a growing appreciation that Inflammatory Bowel Disease (IBD) needs a personalized approach to treatment so that the right therapy can be given to the right patient at the right time. Indeed, the potential benefits of personalized medicine for IBD are evident. One approach may be through prognostic information, which is essential for clinical decision-making, as it provides physicians with substantial evidence that can assist them in guiding patients during their disease and treatment course. Prognostic markers, such as clinical, serological, endoscopy, fecal, and histology factors, predict the natural course of the disease, and their utility is based on the assumption that treatment stratification can impact the course of the disease. Therefore, recent literature has focused on identifying good prognostic models based on genetic/serological or clinical/demographic factors that could substantially contribute to tailoring each patient's treatment and potentially surpass a significant barrier to personalized medicine in IBD due to the lack of algorithms to guide treatment from diagnosis.
This talk will present and summarize the most recent scientific evidence of disease risk factors as prognostic tools in Crohn's disease (CD) and ulcerative colitis (UC) and provide some take-home messages on this topic such as:
- Patients with CD and UC are so heterogeneous that a single treatment algorithm will never be suitable. Therefore, we need individualized treatment options and decisions.
- System dynamics analysis is a methodology that addresses the inherent dynamic complexity of interactions between variables. As an alternative to traditional statistical methods, it may have the ability to translate complex clinical data into patient-friendly results.
- Bayesian networks can be seen as an alternative to logistic regression, where statistical dependence and independence are not hidden in approximating weights but rather explicitly represented by links in a network of variables.
Compared to the general population, patients with (IBD) have a well-recognized increased risk of developing dysplasia and/or colorectal cancer (CRC), both in ulcerative colitis (UC) and Crohn’s disease (CD). Chronic inflammation is believed to promote the development of neoplasia. Adenocarcinoma complicating ulcerative colitis and Crohn’s disease develops from a precursor lesion, dysplasia or intra epithelial neoplasia, via an inflammation-dysplasia-carcinoma sequence. IBD-related dysplasia is categorized into either low-grade or high-grade dysplasia. Until recently, dysplasia was classified macroscopically into two general categories: flat dysplasia, which is endoscopically undetectable, and elevated or raised dysplasia. In 2015, the SCENIC [Surveillance for Colorectal Endoscopic Neoplasia detection and management in inflammatory bowel disease patients: International Consensus] nomenclature proposed the classification of lesions as visible or invisible to guide its clinical management. There is some evidence that flat/invisible dysplasia in IBD may have different molecular features when compared with polypoid/visible dysplasia. Adding to this complexity, several different histologic patterns of non-conventional dysplasia in IBD have been recently described that are morphologically distinct from conventional (or intestinal-type) dysplasia. At least seven subtypes have been reported, including, (i) hypermucinous; (ii) goblet cell-deficient; (iii) crypt cell dysplasia (or dysplasia with terminal epithelial differentiation); (iv) dysplasia with increased paneth cell differentiation; (v) sessile serrated lesion-like dysplasia; (iv) TSA-like; (v) ; (vi) (vii) serrated dysplasia, not other specified. There is increasing evidence that some of these non-conventional dysplasias are high-risk markers for advanced neoplasia. However, there is limited information regarding their clinicopathologic features and clinical outcomes, in part due to the rarity of these subtypes and the likelihood that they are under-recognized
Objective evaluation of treatment response is the gold standard in ulcerative colitis (UC). In this setting, intestinal ultrasound (IUS) is a non-invasive alternative to endoscopy. Recent studies showed change in IUS parameters after treatment initiation but studies with an endoscopic reference standard are scarce. The aim of this study was to evaluate early change of IUS parameters and determine cut-off values for endoscopic endpoints in UC patients starting anti-inflammatory treatment.
MethodsIn this longitudinal prospective study consecutive patients with moderate-severe UC (baseline endoscopic Mayo score (EMS)≥2) starting an anti-inflammatory treatment were included. Clinical scores, biochemical parameters and IUS parameters were collected at baseline, after 2 (T1), 6 (T2) and 8-26 weeks (T3) around time of the second sigmoidoscopy/colonoscopy. IUS parameters were measured as previously established1. Endoscopic remission (ER) and mucosal healing (MH) were evaluated in the sigmoid and defined as EMS=0 and EMS≤1, respectively. The ultrasonographist and endoscopist were blinded for the outcomes of endoscopy and IUS, respectively.
Results51 consecutive patients were included (Table 1) of whom 31 underwent a second endoscopy (MH: n=15 (45%), ER: n=9 (27%)). Two additional patients underwent colectomy and were considered non-responders. 18 patients did not undergo second endoscopy due to the COVID-19 pandemic (n=2), refusal (n=5), loss to follow-up (n=1) or treatment escalation because of clinical deterioration confirmed by IUS and biomarkers before second endoscopy was performed (n=10). Bowel wall thickness (BWT) was significantly lower from T2 onwards in patients reaching MH (p=0.026) and ER (p=0.002) at T3 (Fig 1). A significant decrease in BWT was already visible at T1 in patients receiving infliximab (p=0.001) or tofacitinib (p=0.007), but not in patients treated with vedolizumab (p=0.11) (Fig 2). Most accurate BWT cut-off values at T3 to determine MH and ER were 3.52 mm (AUROC: 0.95, 95% CI: 0.86-1.00, p<0.0001, sens: 91%, spec: 91%) and 2.98 mm (AUROC: 0.94, 95% CI: 0.85-1.00, p=0.001, sens: 87%, spec: 100%), respectively. Other IUS parameters at T3 did not improve association with MH or ER. IUS parameters at T2 that predict MH and ER are demonstrated in Table 2.
Table 1
Fig 1

Fig 2
 Table 2
Table 2Conclusion
BWT and Colour Doppler Signal 6 weeks after start of treatment are associated with and could predict MH and ER. In addition, treatment response patterns at IUS are drug-specific. Furthermore, we have provided accurate BWT cut-off values for endoscopic outcomes. In a point-of-care setting, (early) treatment evaluation with IUS could guide treatment decision in UC in order to optimize treatment response.
1. Bots et al, JCC, 2021
Summary:
This presentation summarises the second ECCO Guideline on Malignancy in IBD. The guideline includes 24 statements, each underpinned by a systematic review of the literature. The topics covered include risk of cancer in IBD, malignancy risk associated with small molecule and biological therapies, and management of IBD in patients with a history of cancer.
Of course, this is too much to cover in a single presentation. As such, we will focus on the key developments since our previous guidance, as outlined below.
Educational objectives:
1) To recap prevention of colorectal cancer in IBD, with a focus on
- colorectal cancer screening and surveillance
- detection and management of dysplasia in IBD
2) To review IBD therapy and associated cancer risk
3) To understand how best to approach managing IBD in patients with active or recent malignancy
Educational objectives
- To introduce the updated ECCO guidelines on IBD and malignancy
- To highlight the latest evidence on colonoscopy surveillance in IBD
- To discuss the malignancy risk of specific therapies in IBD
- To discuss the management of patients with IBD and active malignancies
Education objectives
1. To present the updated ECCO consensus on Sexuality, Fertility, Pregnancy and Lacation
2. To discuss pregnancy and IBD
3. To review safety of drugs during pregnancy and lactation in IBD
New ECCO pregnancy guideline
1. Pre conception counseling, optimal disease control, planning, adherence
2. Drug safety at conception and during pregnancy
3. Management of disease exacerbation during pregnancy, assessment and therapeutic options
4. Management of biologics during pregnancy and post-partum
5. Multidisciplinary decision concerning through the entire pregnancy and important decision like mode of delivery
1. to present the new ECCO guidelines on therapeutic in ulcerative colitis
2. to present for acute severe ulcerative colitis, both medical and surgical management
3. to discuss Medical Versus Surgical Management of Refractory Moderate-to-severe UC
4. to give an overview on Preoperative Optimisation of Refractory Moderate-to-severe UC
5. to discuss Surgical Strategy and technical surgical aspects of Refractory Moderate-to-severe UC
Educational objectives
- To discuss the new ECCO guidelines on ulcerative colitis
- To consider the role of GRADE methodology in driving guideline quality
- To highlight relevant sections of the medical and surgical guidelines that may inform practice
A European IBD Voyage
Séverine Vermeire
University Hospital Leuven Belgium
Ever since the early foundations of ECCO, groundbreaking discoveries in IBD have significantly impacted on how we look at the disease and approach IBD in the clinic today. Many of these discoveries not only had their origin in Europe but also laid the foundations for collaboration across and beyond our continent. The parallel expansion and diversification of ECCO embraced these discoveries, and facilitated further growth towards improvements for patients and patient care.
One early landmark discovery was the familial aggregation and increased disease concordance among monozygotic twins, observations first described in Scandinavian cohorts but later confirmed by many, and pointed towards a strong genetic susceptibility. Soon, the first genome-wide linkage and later association scans were a fact and the international IBD Genetics Consortium, born in Oxford, UK was the start of almost 25 years of collaborative research culminating in the discovery of the NOD2/CARD15 gene in 2001 and many other important findings such as the role of autophagy and innate immunity. Another groundbreaking discovery originally described by teams from London, UK and Hannover, Germany, was the recognition of monogenetic Very Early Onset (VEO) IBD. The VEO-IBD Consortium now has global participation and provides guidelines for the work up and management of these complex patients. VEO-IBD research has been pivotal in unravelling disease pathways such as general immune dysregulation, T and B cell defects, phagocytic defects, hyper- and auto-inflammatory conditions and epithelial barrier dysfunction.
Incidence trends of disease across Europe from North to South were put on the map by the EC-IBD study group and were later expanded from West to East by the EpiCom collaborative study Group which ECCO fostered. Till today, these epidemiological trends are crucial to help in deciphering disease pathogenesis and the role of the exposome.
Collaboration between the gastroenterology, abdominal surgery and pathology departments in Leuven, Belgium in the late eighties and nineties on postoperative Crohn’s recurrence showed the importance of faecal stream diversion and antibiotics in preventing postoperative relapse. This in turn triggered interest in the role of the microbiome with pioneer studies from Berlin, Germany and in antimicrobial serology such as ASCA in the early stages of disease; research which took off in Lille, France and rapidly spread globally. In later years the role of the mesenteric fat in disease pathogenesis was recognized and closer collaboration between surgeons and IBD pathologists within ECCO was born. Till today, no effective postoperative prevention exists but new research tools including single cell and spatial transcriptomics and metabolomics bring hope.
European groups such as the French GETAID, the Leuven, Barcelona and Nancy IBD Centers have pioneered clinical research in development of endoscopic, radiologic and histologic scores for disease severity such as the CDEIS, SES-CD, Rutgeerts score, Maria and Nancy index. Almost 30 years later, new technologies such as machine learning and Artificial Intelligence are paving the way for a more accurate disease classification, and molecular methods have started to create a dream that disease clearance may become reality soon.
In conclusion, several important advances in disease pathogenesis, but also in developing better tools to diagnose, score and treat IBD were made in Europe. Although many challenges remain, new tools and techniques now make it possible to provide answers, together with the help of the many patients and the agility of ECCO.Upadacitinib (UPA) is an oral, once-daily (QD), selective Janus kinase inhibitor that has demonstrated efficacy in the induction and maintenance treatment of Ulcerative Colitis (UC). This analysis aimed to assess the impact of baseline UC characteristics on clinical outcomes following UPA therapy.
MethodsPatients (pts) with moderately to severely active UC who had failed conventional or biological treatment were enrolled in two randomised, double-blind (DB), placebo (PBO)-controlled, Phase 3, 8-week induction studies of UPA 45 mg QD vs PBO. Pts achieving a clinical response (decrease from baseline in Adapted Mayo score ≥2 points and ≥30% from baseline, plus a decrease in rectal bleeding score [RBS] ≥1 or an absolute RBS ≤1) at Week (Wk) 8 entered a 52-week, randomised, DB, PBO-controlled, Phase 3 maintenance study, and received UPA 15 mg QD, UPA 30 mg QD, or PBO. The primary efficacy endpoint was clinical remission per Adapted Mayo score at Wk 8 (induction) and at Wk 52 (maintenance). Pre-specified (PS) and post hoc (PH) analyses were conducted by baseline disease activity (full Mayo score ≤9 or >9) (PS), extent (PS), duration (<2, 2–5, >5–10, >10 years [yrs]) (PH), and high-sensitivity C-reactive protein (hs-CRP) level (≤5 or >5 mg/L) (PH).
Results988 pts were included in the induction studies, and 451 in the maintenance study. The treatment difference between UPA 45 mg QD and PBO excluded zero for all subgroups at Wk 8 (Figure 1). Wk 8 remission rates were lower for full Mayo score >9 vs ≤9, and for CRP >5 vs ≤5 mg/dL, with minimal effects of disease extent or duration. Wk 52 maintenance of remission rates were greater with UPA 15 mg and 30 mg QD vs PBO (16–44% and 30–50% difference, respectively), and with UPA 30 vs UPA 15 mg QD. This incremental benefit of the 30 mg dose was greater in pts with severely (full Mayo score >9) vs moderately active disease (Figure 2); 15 mg and 30 mg demonstrated similar efficacy in pts with Mayo score <9. Statistically non-significant numerical advantages for UPA 30 mg vs UPA 15 mg maintenance were seen in all subgroups, with the exception of hs-CRP >5 mg/L.

UPA 45 mg QD is an effective induction treatment for UC, regardless of baseline disease characteristics. Both 15 and 30 mg UPA doses are effective maintenance regimens, regardless of baseline disease characteristics. However, the 30 mg dose shows a trend towards increased benefit vs 15 mg in pts with Mayo score >9 and extensive disease. This analysis suggests that while UPA 15 mg may be most appropriate for patients with UC with a low inflammatory burden (per full Mayo score and extent), the 30 mg dose may be more appropriate for those with a high one.
EIMs are common in patients with UC and are associated with impaired quality of life. This analysis evaluated the impact of treatment with the selective Janus kinase 1 inhibitor UPA on EIMs in patients with moderate-to-severe UC.
MethodsData were included from two induction studies (U-ACHIEVE Induction [NCT02819635] and U-ACCOMPLISH [NCT03653026]) and a maintenance study (U-ACHIEVE Maintenance). Patients with moderate-to-severe UC were randomised 2:1 to 8 weeks’ induction treatment with UPA 45 mg once daily (QD) or placebo. Patients with a clinical response to induction were re‑randomised (1:1:1) to 52 weeks’ maintenance treatment with UPA 15 mg or 30 mg QD, or placebo. The presence of EIMs (peripheral arthropathy, axial arthropathy, episcleritis, uveitis, iritis, erythema nodosum, pyoderma gangrenosum, Sweet’s syndrome, oral aphthous ulcers, primary sclerosing cholangitis, autoimmune hepatitis, venous thromboembolism, chronic obstructive pulmonary disease, bronchiectasis, nephrolithiasis and anaemia) was captured in the EIM form at the start of induction (baseline) and at every visit up to Week 52. The induction study results were pooled for analysis.
ResultsAt baseline, 25.0% and 26.5% of patients in the UPA 45 mg QD and placebo induction groups had ≥1 EIM, while 24.3%, 26.6% and 24.8% of patients randomised to maintenance treatment with UPA 15 mg QD, UPA 30 mg QD or placebo, respectively, had ≥1 EIM (Table 1). The most common EIMs at baseline were anaemia, peripheral arthropathy and axial arthropathy; all other EIMs were reported in <2% of patients (Table 1). The proportion of patients reporting resolution of any EIM, arthropathy (peripheral and axial) and anaemia at Week 8 was numerically greater with UPA 45 mg QD than placebo in the induction studies (Figure 1A). Resolution at Week 52 of any EIM in patients with ≥1 EIM at baseline was significantly increased with UPA 30 mg QD (p<0.001), and numerically greater with UPA 15 mg QD, versus placebo (Figure 1B). In patients with arthropathy (peripheral or axial) at baseline, the proportion achieving resolution of arthropathy (peripheral and axial) at Week 52 was significantly increased with UPA 30 mg QD (p=0.010), and numerically higher with UPA 15 mg QD, versus placebo (Figure 1B). The same was true of anaemia resolution in patients with anaemia at baseline (p=0.019 for UPA 30 mg QD versus placebo; Figure 1B).

UPA treatment is effective in resolving EIMs in patients with UC. EIM symptom resolution was improved versus placebo following induction treatment with UPA 45 mg and after maintenance treatment with UPA 15 or 30 mg, with the 30 mg dose providing statistically significant improvements versus placebo.
Background
Clinicians face difficulty in positioning biologics and JAK inhibitors in anti-TNF refractory ulcerative colitis (UC) patients. Head-to-head trials comparing the efficacy of vedolizumab and tofacitinib in UC patients are lacking. We aimed to compare the effectiveness and safety of vedolizumab and tofacitinib in anti-TNF experienced UC patients in our prospective, nationwide registry using a propensity score weighted cohort.
Methods
UC patients who failed anti-TNF treatment (with or without thiopurine) and initiated vedolizumab or tofacitinib treatment subsequently, were identified in the observational prospective Initiative on Crohn and Colitis (ICC) Registry. We selected patients with both clinical (Simple Clinical Colitis Activity Index (SCCAI) >2) and biochemical (C-reactive protein (CRP) >5mg/L or faecal calprotectin (FC) >250 µg/g) or endoscopic disease activity (endoscopic MAYO score ≥ 1) at initiation of therapy. Patients previously treated with vedolizumab or tofacitinib were excluded. Corticosteroid-free clinical remission (SCCAI<2), biochemical remission (CRP ≤5 mg/L and/or FC ≤250 µg/g) and safety outcomes were compared after 52 weeks of treatment. Inverse propensity scores weighted comparison was used to adjust for confounding and selection bias.
Results
Overall, 83 vedolizumab and 65 tofacitinib treated patients were included (table 1). Propensity score weighted analysis showed that tofacitinib treated patients were more likely to achieve corticosteroid-free clinical remission at week 12, 24 and 52 compared to vedolizumab treated patients (OR: 5.87, 95%CI:3.55-9.70, P<0.01, OR: 2.96, 95%CI: 1.85-4.73, P<0.01 and OR 2.96, 95%CI: 1.85-4.73, P<0.01, respectively) (table 2). In addition, tofacitinib treated patients were more likely to achieve biochemical remission at week 12 and week 24, remaining only statistically borderline at week 52 (OR: 2.96, 95%CI: 1.85-4.73, P<0.01, OR: 2.96, 95%CI: 1.85-4.73, P<0.01 and OR 1.68, 95%CI: 0.99-2.86, P=0.05, respectively) (table 2). There was no difference in infection rate (OR:1.057, 95%CI: 0.60-1.86, p=0.85) or severe adverse events (OR: 0.39, 95%CI: 0.03-4.33, P=0.44). No thromboembolic events were observed. Most common reason for treatment discontinuation was loss of response (table 3).


Conclusion
In tofacitinib treated, anti-TNF experienced, UC patients, we observed that a higher proportion of patients achieved corticosteroid-free remission after 12, 24 and 52 weeks compared to vedolizumab treated patients. In addition, more tofacitinib treated patients achieved biochemical remission at week 12 and 24. There was no statistically significant difference in severe adverse events.
The therapeutic armamentarium to treat adult patients with moderately to severely active ulcerative colitis (UC) continues to evolve. With this rapid innovation, the comparative efficacy and safety of more recent advanced therapies remain unknown.
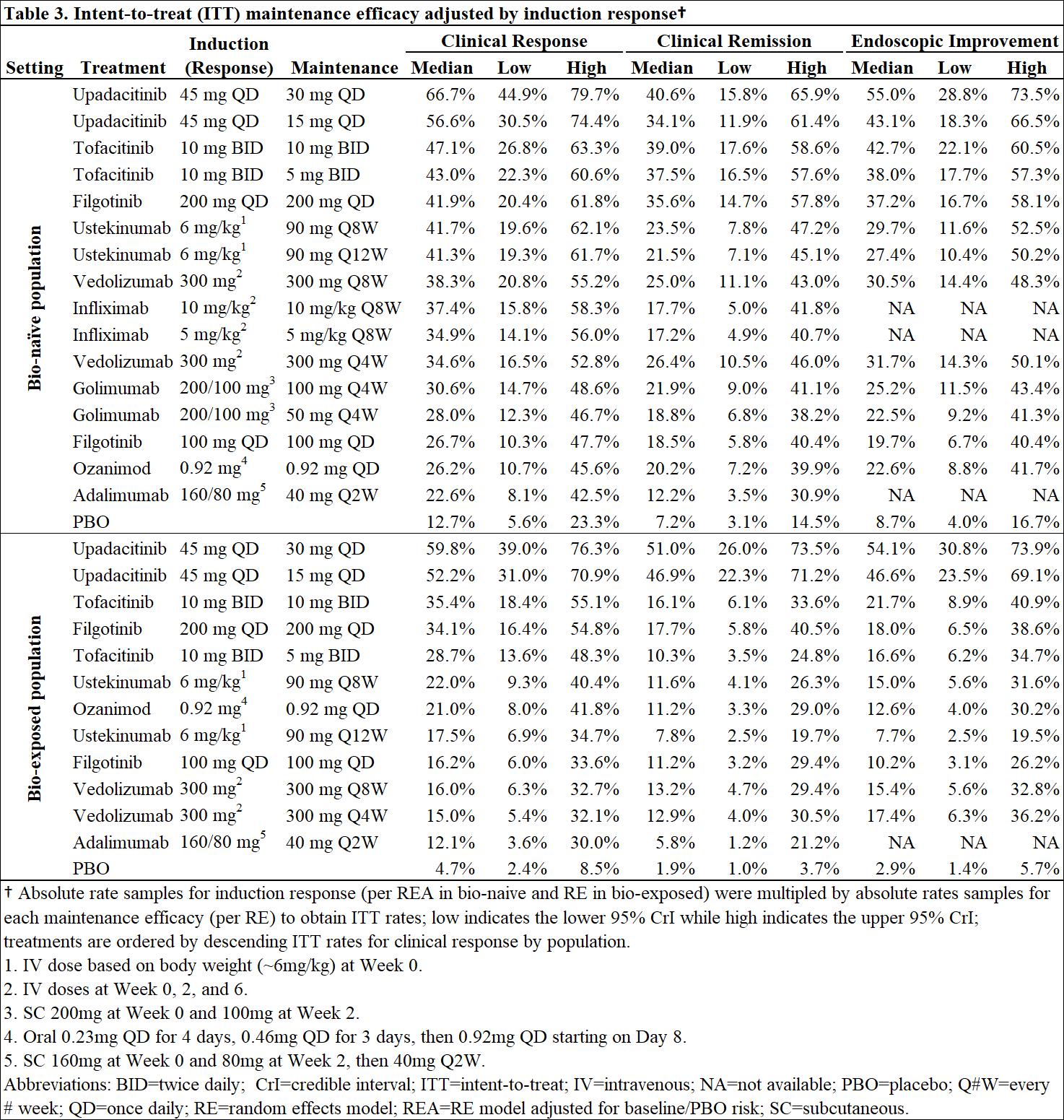
MethodsBayesiannetwork meta-analysis was used to indirectly compare the efficacy and safety of advanced therapies for induction (6-10 weeks) and maintenance (44-54 weeks post-induction response) in adults with moderately-to-severely active UC. Efficacy was assessed separately in bio-naïve and bio-exposed populations by clinical remission (Full Mayo score [FM] of ≤2 with no subscore >1), clinical response (decrease from baseline in FM ≥3 points and ≥30% with decrease in rectal bleeding score [RBS] of ≥1 or absolute RBS ≤1) and endoscopic improvement (endoscopic score ≤1); ad hoc analyses were conducted on upadacitinib (UPA) RCT data to produce FM outcomes. Safety was assessed by discontinuation due to adverse events (AEs), serious AEs, and serious infections. Induction therapies included UPA 45 mg, adalimumab 160/80 mg, filgotinib 100 and 200 mg, golimumab 200/100 mg, infliximab 10 and 5 mg/kg, ozanimod 0.92 mg, tofacitinib 10 mg, ustekinumab (UST) 6 mg/kg, and vedolizumab (VED) 300 mg. The maintenance analysis included low and high maintenance doses of these therapies. Phase 3 randomized controlled trials (RCTs) were identified via systematic literature review. Random effects models were used to account for expected heterogeneity in endpoints and study design. Guidelines from the National Institute for Health and Care Excellence were followed.
ResultsOut of 31 RCTs identified, 23 were included (18 for induction and 14 for maintenance). Odds ratios vs. placebo (PBO), numbers needed to treat/harm, and surface under the cumulative ranking curve estimates are presented for efficacy in bio-naïve (Table 1) and bio-exposed (Table 2) populations and for safety in overall populations (Table 4). Intent-to-treat rates of maintenance efficacy outcomes adjusted by the likelihood of induction response show UPA to be consistently the most efficacious therapy (Table 3). There were no significant differences in serious AEs or serious infections for any advanced therapy vs PBO. For discontinuation due to AEs, only UPA had significantly lower odds vs PBO after induction, while UST and VED had significantly lower odds vs PBO after maintenance (Table 4).
ConclusionIn patients with moderately-to-severely active UC, UPA 45 mg induction and 30 mg maintenance appear more efficacious than other advanced therapies/PBO at inducing and maintaining clinical response, clinical remission, and endoscopic response, with no greater safety assessments vs PBO, over 1-year.


Anoperianal lesions affect up to 30% of patients with Crohn’s disease (CD). Long-term fistula healing is challenging with conventional biotherapies. Although recent studies demonstrated the efficacy of local injections of adipose tissue-derived stem cells with 50 % of fistulae closure without abscess at one year, this treatment is not available in routine.
The primary aim of this study was to evaluate the safety and the feasibility of the injection of bone marrow-derived mesenchymal stem cells isolated and prepared in a local university laboratory of cell therapy for perianal fistulizing CD. The second aim was to evaluate the efficacy of this treatment and his impact on the quality of life of the patients.
MethodsA prospective observational study was performed in the CHU of Liège from October 2019 till October 2021. All CD patients with perianal fistula and seton placement for at least 6 months were eligible. PRO, clinical examination, CRP, fecal calprotectine, CDAI, Short health scale (SHS) and MRI were performed at weeks 0, 12 and 48. PDAI was calculated at inclusion and at Week 48. Efficacy was defined as closure of all treated external openings at clinical examination without abscess at MRI.
ResultsSixteen patients with a median age of 49 years old and a median duration of perianal CD of 8 years were included. Eleven (69%) patients were on anti-TNF. CDAI and PDAI at inclusion were 97,5 ± 48,8 et 5 ± 4,4 respectively. Four (25%) patients reported adverse events the week after the injection (local pain 3/16, mild bleeding 1/16), all of them quickly resolutive. Ten (63%) and 8 (50%) patients had a closure of all the external opening at week 12 and 48 respectively. Five out of 6 patients with 2 external openings had at least 1 opening closed at Week 48. One abscess was observed during the follow-up. The median PDAI was numerically lower at the end of the study (3 versus 5 at the inclusion). The quality of life improved with a regression of the SHS from 10 to 7.5 at the end of the follow-up. At MRI, MAGNIFI-CD score and Van Assche index were similar for each patients at the inclusion and at the end of the study.
ConclusionInjection of locally prepared bone marrow-derived mesenchymal stem cells seems safe and effective in refractory perianal fistulae in Crohn’s disease with 50% of closure at 1 year. The treatment is associated with an improvement of the perianal activity scores and the quality of life scores but not with the MRI scores.
Preclinical data from a murine model of acute colitis suggest that dual blockade of interleukin(IL)-23 and TNFα more effectively prevented the development of colonic inflammation than each monotherapy. Guselkumab(GUS), an IL-23p19 subunit antagonist, is being studied in inflammatory bowel disease. Golimumab(GOL), a TNFα antagonist, is approved for ulcerative colitis(UC). The objective of this study was to evaluate the efficacy and safety of combination induction therapy with GUS+GOL vs GUS or GOL alone in adults with moderately-to-severely active UC.
214 patients(pts) naïve to TNFα antagonists and refractory or intolerant to conventional therapy(ie, immunomodulators and/or corticosteroids) were randomly assigned to receive GUS 200mg intravenous(IV) at weeks(wks) 0, 4, and 8(n=71); GOL 200mg subcutaneous(SC) at wk0 then 100mg SC at wks2, 6, and 10(n=72); or combination with GUS 200mg IV+GOL 200mg SC at wk0, GOL 100mg SC at wks2, 6, and 10, and GUS 200mg IV at wks4 and 8(n=71). The primary endpoint was clinical response at wk12; the major secondary endpoint was clinical remission at wk12. Other key endpoints were clinical remission based on the modified Mayo score (mMayo), symptomatic remission, endoscopic improvement, endoscopic normalization, histologic remission, composite histologic-endoscopic endpoints, and biomarker outcomes.
ResultsBaseline disease characteristics were similar among groups(Table 1), however a greater proportion of pts in both monotherapy groups had pancolitis vs the combination group. A greater proportion of pts who received combination therapy achieved clinical response at wk12(83.1%) vs GUS(74.6%) or GOL(61.1%)(Table 2). Similarly, the proportion of pts who achieved clinical remission in the combination group(36.6%) was greater than that of monotherapy groups(21.1% and 22.2%, respectively). Clinical remission by mMayo score, endoscopic improvement, histologic remission, both histologic remission and endoscopic improvement, and biomarker normalization (calprotectin, CRP) rates at wk12 were also greater in the combination group vs GUS or GOL. Percentages of pts with endoscopic normalization and both histologic remission and endoscopic normalization were nearly double with combination therapy vs either monotherapy. Adverse event(AE), serious AE, and infection rates were comparable among treatment groups. One pt receiving combination therapy experienced a serious infection of influenza and sepsis. No deaths, malignancies, or TB cases were reported through wk12.

Combination induction treatment with GUS+GOL more effectively induced clinical response, clinical remission, and endoscopic improvement at wk12 than either monotherapy alone. AE rates were comparable among the treatment groups.
Tyrosine kinase 2 (TYK2) is an intracellular kinase that mediates the signalling of key cytokines involved in ulcerative colitis (UC) pathophysiology. Deucravacitinib (DEUC) is a novel, oral, selective TYK2 inhibitor that binds to the TYK2 regulatory domain. The safety and efficacy of DEUC were evaluated in patients (pts) with moderately-to-severely active UC.
MethodsLATTICE-UC (NCT03934216), a randomised, double-blind, placebo (PBO)-controlled, multicentre, Phase 2 trial, enrolled pts with moderately-to-severely active UC (modified Mayo score of 5 to 9 [endoscopic {ES} subscore ≥2, rectal bleeding {RB} subscore ≥1, stool frequency {SF} subscore ≥2) with inadequate response, loss of response, or intolerance to ≥1 conventional or biologic therapy. Pts were randomised 2:1 to oral DEUC 6 mg or PBO twice daily (BID) and stratified by baseline (BL) corticosteroid use and prior exposure to biologics. The primary endpoint was clinical remission (modified Mayo score with subscores of SF ≤1 with ≥1-point decrease from BL, RB=0, and ES ≤1 [modified, excludes friability]) at Week (Wk) 12; endoscopic response (ES ≤1) at Wk 12 was a secondary endpoint.
ResultsDemographic and BL characteristics were generally similar across groups, except for BL disease activity as measured by the modified Mayo score and ES subscore. Most pts (63.4%) were biologic naïve, and 40.5% were receiving concomitant oral corticosteroids (Table 1). Of 131 pts randomised, 104 (79.4%) completed 12 wks of treatment (DEUC, 69/87 [79.3%]; PBO, 35/42 [83.3%]). At Wk 12, clinical remission rates were 14.8% and 16.3% in the DEUC and PBO arms, respectively, in the overall population (P=0.59); 14.0% and 25.9% in biologic-naïve pts; and 16.1% and 0.0% in biologic-experienced pts (Figure 1). At Wk 12, endoscopic response rates were 19.3% and 27.9% in the DEUC and PBO arms, respectively, in the overall population (P=0.88); 15.8% and 37.0% in biologic-naïve pts; and 25.8% and 12.5% in biologic-experienced pts (Figure 2). Pharmacodynamic data suggest insufficient inhibition of TYK2 pathways with DEUC 6 mg BID. Incidence of adverse events (AEs) was 70.1% in the DEUC arm and 47.6% with PBO; rates of serious AEs were 9.2% (n=8) and 4.8% (n=2), respectively. Rash, acne, and worsening of UC were the most common AEs in the DEUC arm. No meaningful changes from BL in mean values of laboratory parameters were observed with DEUC treatment.
This Phase 2 study of DEUC 6 mg BID in moderately-to-severely active UC did not meet its primary or secondary efficacy endpoints at Wk 12. In biologic-experienced pts, response rates were numerically higher with DEUC compared with PBO. The safety profile was consistent with DEUC trials in psoriasis and psoriatic arthritis.


Upadacitinib (UPA), a selective and reversible Janus kinase inhibitor, has been shown to be safe and effective when administered at a dose of 45 mg once daily (QD) as 8-week induction therapy in moderate-to-severe Ulcerative Colitis (UC). This analysis evaluated outcomes following extended induction (45 mg QD for 16 weeks) followed by maintenance (15 or 30 mg QD) treatment with UPA in patients with UC who did not achieve a clinical response after 8 weeks’ induction.
MethodsPatients with moderate-to-severe UC who failed to achieve a clinical response (Adapted Mayo score decrease of ≥2 points and ≥30% from baseline, plus ≥1-point decrease in rectal bleeding score [RBS] or absolute RBS ≤1) to 8 weeks’ induction treatment with UPA 45 mg QD in the U-ACHIEVE Induction (NCT02819635) or U‑ACCOMPLISH (NCT03653026) studies, continued to receive UPA 45 mg QD in an 8‑week open-label extension. Responders at the end of the open-label extension entered the U-ACHIEVE Maintenance study and were randomised 1:1 to UPA 15 mg or 30 mg QD for 52 weeks. The efficacy endpoints were evaluated at Week 16 for the induction studies and at Week 52 for the maintenance study.
ResultsIn total, 125 patients who failed to achieve a clinical response after 8 weeks’ induction treatment received open-label UPA 45 mg for a further 8 weeks. Of these patients, 48.3% achieved a clinical response at Week 16 and were re‑randomised to UPA 15 or 30 mg in U-ACHIEVE Maintenance. Among 16-week responders who entered the maintenance study, clinical remission, maintenance of clinical response, and endoscopic improvement, respectively, at Week 52 were achieved in 33.3% versus 19.0%, 66.7% versus 35.7%, and 37.5% versus 23.8% of those who received UPA 30 versus UPA 15 mg QD as maintenance treatment (Table 1). Adverse events of special interest were reported infrequently in the two maintenance treatment groups (Table 2).

In this analysis, prolonged induction treatment for a total of 16 weeks was beneficial in almost half of UC patients who failed to achieve a clinical response after 8 weeks’ induction with UPA 45 mg. The benefit of maintenance therapy in these delayed responders was further demonstrated, with UPA 30 mg providing greater benefit than UPA 15 mg QD.
Treatment of perianal fistulizing Crohn’s disease (PFCD) is a major unmet need. Filgotinib (FIL) is a once-daily, oral, preferential Janus kinase 1 inhibitor in development for the treatment of inflammatory bowel diseases. The efficacy and safety of FIL for the treatment of PFCD was evaluated in the phase 2, double-blind, randomized, placebo (PBO)-controlled DIVERGENCE 2 study (NCT03077412).
MethodsPatients (18–75 years old) with PFCD (documented diagnosis of CD for at least 3 months and 1–3 external openings [EOs] with drainage [spontaneous or on compression] for ≥ 4 weeks before screening) previously treated with antibiotics, immunomodulators and/or tumour necrosis factor inhibitors (TNFi) were randomized (2:2:1) to receive FIL 200 mg, FIL 100 mg or PBO once daily for up to 24 weeks. Active luminal CD was permitted providing that the Crohn’s Disease Activity Index score was ≤ 300 at screening. The primary endpoint was combined fistula response (reduction of ≥ 1 from baseline in the number of draining EOs determined by investigator assessment and no fluid collections > 1 cm on centrally read pelvic magnetic resonance imaging [MRI]) at Week 24. Combined fistula remission (closure of all draining EOs present at baseline and no fluid collections > 1 cm) at Week 24 was a key secondary endpoint. The study was not powered for statistical comparisons and was prematurely terminated owing to low recruitment rates during the COVID-19 pandemic.
ResultsBaseline characteristics were broadly similar across the treatment groups (Table 1). Overall, 91.2% of patients had complex perianal fistulae and TNFi treatment had previously failed in 64.9% of patients. A lower proportion of patients randomized to receive FIL 200 mg discontinued the study compared with those who received PBO (Table 2). The proportion of patients who achieved a combined fistula response at Week 24 was numerically higher in the FIL 200 mg group (47.1%; 90% confidence interval [CI]: 26.0–68.9) than in the PBO group (25.0%; 90% CI: 7.2–52.7) (Figure 1), with similar results observed for combined fistula remission (FIL 200 mg [47.1%; CI: 26.0–68.9] versus PBO [16.7%; CI: 3.0–43.8]) (Figure 2). Treatment-emergent severe adverse events were highest in the FIL 200 mg group (Table 2). Adverse event rates were otherwise similar across treatment groups.



In this phase 2 study, numerically higher fistula response and remission rates were observed after 24 weeks of treatment with FIL 200 mg versus PBO in patients with active PFCD and a history of multiple medical treatment failures. FIL was well tolerated overall. Further studies of FIL for the treatment of PFCD are warranted.
