Two new drugs have recently been approved by the European Medical Agency and one additional strategy will soon follow. With regard to the first part, this includes Filgotinib, an addition to the Janus Kinase inhibitor class with a higher specificity to Jak1. Furthermore, an entire new class has entered the field of IBD by the introduction of Ozanimod the first S1P receptor modulator. Last, anti-IL-23 antibodies are expected to be approved soon. The data of the clinical studies will be shortly summarized and all three drugs will be placed in our current treatment algorithm by discussing efficacy, side-effects as well as the role with regard to extraintestinal manifestations.
Educational Objectives:
1. To understand the mode of action of the three drugs described
2. To review the clinical trials that resulted in the approval of these drugs.
3. To know potential side-effects and required strategies.
4. Identify a possible role within the treatment algorithm.
An overview of the use of 5-ASA, steroids and immunomodulators in IBD
- Role and efficacy of Fecal microbiota transplantation in IBD
- Role and efficacy of new nutritional intervention in IBD
Alteration in body composition are common in IBD patients and are often not recognized. Rates of obesity in IBD patients are rising over time, driven by gains in fat mass, while lean mass decreases. As (sarcopenic) obesity can independently predict poor disease outcomes during IBD course, the impact of body composition on the IBD disease course will be studied. In this presentation, we will try to unravel whether obesity can lead to the development of IBD and whether IBD can contribute to obesity.
Current literature predominantly uses BMI as a marker of nutritional status. Since augmentation of body composition parameters in the clinical setting could improve IBD outcomes, it is important that clinicians recognize that an increase in BMI may obscure a decrease in muscle mass. This presentation will finish with some practical treatment advices on body composition in IBD patients such as measurement of bio-impedance or hand grip strength alongside with BMI to predict lean muscle mass.
Educational Objectives:
1. To recognize the prevalence of (sarcopenic) obesity in IBD
2. To review the impact of obesity on the development of IBD
3. To understand the impact of obesity on disease course
4. To have an overview of practical measurement tools to reveal body composition in IBD patients
Educational objectives:
1. To understand the role of 5-ASA in the treatment of IBD and the most frequent mistakes made in 5-ASA treatment
2. To review the evidence for dosing and treatment routes for the different localizations of inflammation in UC and CD
3. To emphasise the role of rectal 5-ASA therapy in proctitis and left sided colitis and the important role of oral/rectal combination therapy
4. To have an overview over optimal treatment strategies with 5-ASA
Inflammatory bowel disease (IBD) are chronic diseases affecting different segments of the digestive tract, also associated with extra-intestinal sites of inflammation. Global research has more intensively focused in developing novel pharmaceutical agents the last 30 years, due to a gradual increase in incidence and prevalence of IBD. 5-aminosalicylic acid (5-ASA) represent one of the older drug classes used for the treatment of IBD patients. The exact mechanism of action has not been elucidated. Proposed mechanisms are: (A) modulation of the inflammatory response originating from the cyclooxygenase and lipooxygenase pathways leading to a decrease of the synthesis of prostaglandins and leukotrienes, (B) interference with the production of inflammatory cytokines via decreasing the activity of nuclear factor κB, and inhibiting tumor necrosis factor and (C) interference with cellular functions of mucosal lymphocytes, macrophages, and natural killer cells. Properties of free radical scavengers and antioxidants have also been postulated. 5-ASA are recommended for the induction and maintenance of remission in mild-to-moderately active ulcerative colitis but are also used in cases with mild Crohn’s disease, although this practice is rather originating from uncontrolled observational data or expert opinion-based and not a formal recommendation in most of the major national or international guidelines. Oral and rectal formulation of 5-ASA exist and the choice of the most appropriate regimen depends on disease extent, localization and severity, the sites of the digestive tract that each formulation is released and patient’s preference. In general, a combination of both formulations is considered the most potent treatment. Safety profile is acceptable with the vast majority of the adverse events being reversible. Monitoring includes principally renal and hepatic function and complete blood count. 5-ASA are relatively fast acting drugs for symptom relief. However, remission may need up to 4-6 weeks to establish. Thus, timely design of optimal treatment strategies with 5-ASA is of utmost importance.
CT-P13 is the first and only subcutaneous (SC) formulation of infliximab (IFX) which received EMA approval in July 2020 for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). This study compares efficacy and safety between IFX SC and vedolizumab (VDZ) in moderate-to-severe CD or UC patients using data from the pivotal study of IFX SC, VISIBLE 2 and recently presented systematic literature review and meta-analysis [1].
This comparative study analyses 7 randomised controlled trials. The IFX SC trial (NCT02883452) was compared with the VDZ trials including GEMINI II, GEMINI III, VISIBLE 2 for CD and GEMINI I, VISIBLE 1, and VARSITY for UC. VISIBLE 2 was added in this analysis as it was published after the presented meta-analysis. In all studied VDZ trials, only responders at week 6 continued to receive maintenance treatment except VARSITY trial, which followed a treat-through design.
Crohn’s Disease Activity Index (CDAI)-70, CDAI-100 response and clinical remission for CD, and clinical response, clinical remission, and mucosal healing for UC at week 6 (induction) and 1 year (week 50–54, maintenance) were compared between IFX SC and VDZ for evaluating efficacy. Discontinuation due to lack of efficacy and safety profiles over 1 year were also assessed.ResultsIn the patients with CD, IFX yielded significantly better efficacy results in the induction phase compared to VDZ with non-overlapping 95% confidential interval while IFX SC displayed better results, although statistically non-significant during the maintenance phase (Table 1). In UC, similar efficacy was shown between the treatments during both induction and maintenance phase (Table 2). The proportion of patients discontinued due to lack of efficacy was significantly higher in VDZ compared to IFX SC in both CD (IFX SC 5% and VDZ 32%) and UC (IFX SC 3% and VDZ 15%) over 1 year (Tables 1,2).
The safety profiles were generally comparable between IFX SC and VDZ. Similar proportion of patients experienced serious adverse event (9% and 14% in CD; 12% and 11% in UC in IFX SC and VDZ). The proportion of patients experiencing serious infection between the treatments was also similar in both CD and UC (Tables 3,4).



Better efficacy was shown in IFX SC compared to VDZ in CD, while a similar efficacy was shown in UC. A significantly higher proportion of patients were discontinued due to lack of efficacy in VDZ compared to that of IFX SC over 1 year. Safety profiles over 1 year were generally comparable between IFX SC and VDZ in both indications.
Reference
[1] Peyrin-Biroulet, L. (2021). P0414 Efficacy and safety of infliximab and vedolizumab in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Poster presented at: 2021 UEG Week.
Evidence suggests there is an increased incidence of inflammatory bowel disease (IBD) in the psoriasis (PsO) population. However prior studies may have underestimated the degree of this association, by not considering (pre-)clinical markers or symptoms of IBD (e.g. elevated faecal calprotectin [FCal] and/or IBD red flags). Furthermore, patients with PsO are not typically screened for IBD. Here we describe the prevalence of IBD indicators in patients with moderate-to-severe PsO.
MethodsThis was a multi-centre UK study of adults (≥18 years) with moderate-to-severe PsO. Patients were recruited from 12 primary care practices in Greater Manchester between November 2019 – April 2021 (in total, 983 eligible patients from an original cohort of 3485 were invited to participate). Retrospective review of primary care medical records was used to confirm a prior IBD diagnosis at baseline. For patients without a confirmed diagnosis, FCal monitoring and a bespoke questionnaire derived from validated measures of gastrointestinal (GI) symptoms and IBD red flags (CalproQuest IBD and Red Flag Index) were used to screen for IBD risk. Baseline interim data are presented as observed proportions, descriptive statistics, and counts along with 95% confidence intervals (CI).
Results252 patients were included: mean age was 57 (±15 SD) years and 54% were female (Table 1). 
Use of non-steroidal anti-inflammatory drug therapy was reported by 15.5% of patients. In total, 64.7% (CI: 53.4−70.6%) of patients had ≥1 indicator of IBD risk at baseline (i.e., ≥1 of: IBD diagnosis, FCal ≥50 ɥg/g or ≥1 IBD red flag) and 3.6% (CI: 1.7−6.7%) of patients had a confirmed diagnosis of IBD. At baseline, 38.1% (CI: 33.5-46.1%) of patients had elevated (≥50μg/g) FCal (Table 2), and 34.9% (CI: 30.2−42.6%) of patients reported ≥1 IBD red flag. Both elevated FCal and GI symptoms were observed in 26.2% (CI: 21.8−33.5%) of patients.
Figure 1 depicts the overlap between different IBD markers in this cohort.
 Conclusion
ConclusionThe risk of IBD in patients with PsO may be higher than previously reported. While 3.6% of patients in this cohort of patients with moderate-to-severe PsO had a confirmed IBD diagnosis, approximately two-thirds of patients exhibited at least one indicator of IBD risk (FCal ≥50μg/g and/or IBD red flag[s]). This study therefore highlights a population for whom further investigation to confirm IBD may be warranted. Prospective follow-up over the next two years will report on the outcomes of investigations, the final diagnoses and treatments in the identified ‘at risk’ cohort.
Background
Histological remission (HR) is evolving as a treatment target in ulcerative colitis (UC). Several histological indices have been developed, however, their widespread adoption beyond clinical trials is limited by practical difficulties and interobserver variability. Furthermore, the relative complexity of available scores hinders the development of an AI algorithm. We aimed to develop a simple histologic index, aligned to endoscopy, and apply it to a computer-aided diagnosis (CAD) system to evaluate HR.
Methods
614 digitalised biopsies (WSI) from 307 UC patients enrolled into a prospective multicentre study1 were analysed. First, the simple PICASSO Histologic Remission Index (PHRI) based only on the presence of neutrophils, was developed and validated by expert pathologists. Table 1
To implement PHRI in a CAD system we designed a semi-supervised inductive transfer learning strategy composed of two modules. The first consists of a novel deep learning strategy based on a convolutional neural network architecture. This detected neutrophils in areas (patches) of a subset of 314 biopsies (172 remission, 142 active), in 158 of which the presence of neutrophils had been meticulously annotated at pixel level. WSI were then divided in training (172), validation (47), and testing sets (95).
Following a multiple instance learning paradigm, a second model combined the features of all patches of each biopsy into a dichotomous result: presence/absence of disease activity. Figure 1 and 2. Finally, we compared the AI prediction with the pathologists’ assessment.
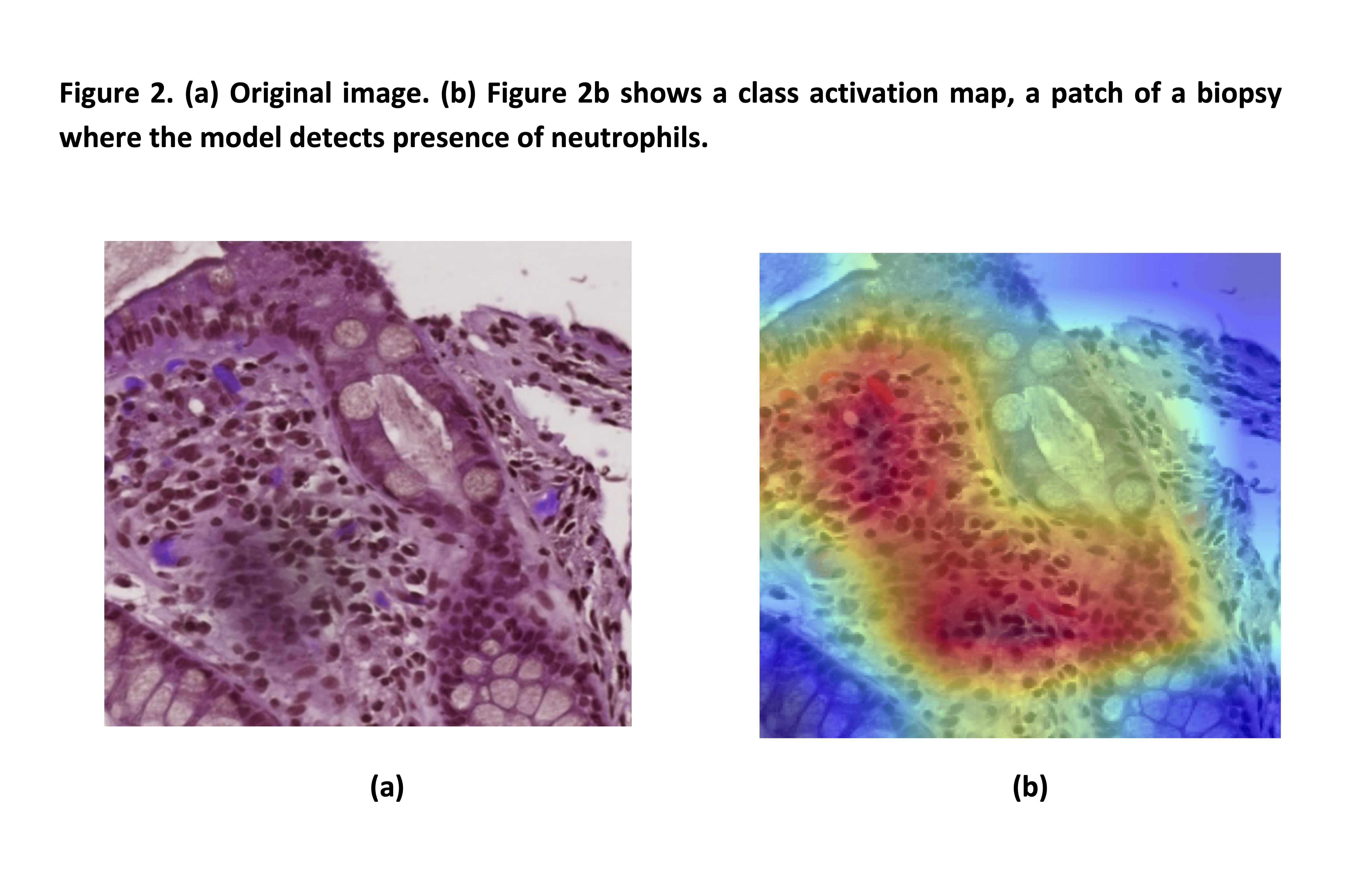

Results
PHRI correlated strongly with all endoscopic scores (MES, UCEIS and PICaSSO) of the same bowel areas (rectum and sigmoid) (Spearman’s ρ= 0.55 to 0.78). Inter-reader agreement between pathologists was almost perfect (ICC 0.84).
In the validation and testing sets our model predicted the presence of neutrophils respectively with 61% and 72% sensitivity, 98% and 84% specificity, 93% and 75% positive predictive value (PPV), and 86% and 83% negative predictive value (NPV) respectively (Table 2).
When predicting remission in whole biopsies, in the validation cohort the AI system had 65% sensitivity, 93% specificity, 86% PPV, and 78% NPV. In the testing cohort the same metrics were 62%, 94%, 90%, and 73% (Table 2).
Conclusions
PHRI is the simplest histological index in UC and correlates strongly with endoscopic activity. Based on PHRI we developed the first artificial intelligence model able to accurately predict histological remission in biopsies of UC. This tool can effectively expedite, support, and standardise the histological assessment of UC in clinical practice.
Reference:
1. Iacucci et al. Gastroenterology 2021
Patients undergoing colorectal surgery for inflammatory bowel disease (IBD) are recognized to have an increased risk of venous thromboembolism (VTE). The aim of this study was to determine the perioperative risk factors for VTE and to create a predictive scoring system for VTE in the IBD cohort.
MethodsThe NSQIP-IBD registry from 2017-2020 was used to identify patients for the study. Demographics, operative and outcomes data of IBD patients undergoing colectomies for IBD were analyzed. Student t and χ2 tests were used for univariate analysis. A logistic multivariate regression model was performed using all significant variables to develop a predictive scoring system of VTE.
Results5003 patients (51.9% male, mean age 42.7, 57.3% Crohn’s / 42.7% ulcerative colitis) were included in the study. 125 (2.49%) developed VTE. The univariate analysis is presented in Table 1. On multivariate analysis ASA grade, ulcerative colitis, sepsis, serum sodium <139 mmol/L, an open abdomen and preoperative inter hospital transfer were associated with greater risk of VTE. An open abdomen postoperatively was associated with the highest odds of developing a VTE [2.69 (1.20 – 5.39, p=.009)]. Using these 6 significant factors, a risk model was created. The risk of VTE with one risk factor was 0.7% and 1.8% with two risk factors. The risk of VTE increased to 3.6% and 4.5% with three and four risk factors respectively. With five and six risk factors, the risk of VTE increased exponentially to 10.9%, 25% respectively (Figure 1).
This study affirms that multiple perioperative and operative factors increase the risk of VTE after surgery for IBD. We present a novel model which demonstrates a cumulative risk increasing exponentially when more than five risk factors are present.
Etrasimod (ETR) is a once-daily, oral, selective sphingosine 1-phosphate receptor modulator in clinical development for immune-mediated inflammatory disorders including ulcerative colitis, Crohn’s disease, eosinophilic esophagitis, and atopic dermatitis. This study evaluated the single-dose bioequivalence of the proposed commercial and clinical formulations of ETR 2mg in the fasted state, and the effect of food on the pharmacokinetics of the proposed commercial formulation.
MethodsThis 3-treatment, 3-period crossover Phase 1 study enrolled 18 healthy adults (18-55 years: 10 males/8 females). Participants were randomized to 6 treatment sequences (3 participants/sequence: ABC, BCA, CAB, ACB, BAC, CBA) consisting of Treatment A (single-dose ETR 2mg clinical formulation, fasted), Treatment B (single-dose ETR 2mg proposed commercial formulation, fasted), and Treatment C (single-dose ETR 2mg proposed commercial formulation, fed). Treatments were administered on Days 1, 10, and 19 to allow 8-day washout periods between treatments. In the fed state (Treatment C), all participants received an FDA-standard high-fat, high-calorie meal. Blood samples for determination of ETR concentration were collected at prespecified timepoints to 168 hours post dose. Analysis of variance was performed on log-transformed Cmax, AUC0-last, and AUC0-∞ values to determine geometric least squares mean ratios (GLSMR) of Test/Reference and associated 90% confidence intervals (CI) to assess bioequivalence and food effects. Safety and tolerability of ETR were also evaluated.
ResultsBioequivalence: The 90% CI for the GLSMR of ETR Cmax and AUC plasma exposure measures, comparing the proposed commercial vs clinical formulations, were within the accepted 80%–125% range (GLSMR and 90% CI) for establishing bioequivalence (Table 1). Median Tmax for Treatments A and B were both 4 hours. Food Effect: The 90% CI for the GLSMR of ETR Cmax and AUC plasma exposure measures, comparing the proposed commercial formulation under fed vs fasted conditions, were within the accepted 80%–125% range for establishing no food effect (Table 2). Median Tmax for Treatment C was 6 hours. Safety and Tolerability: All treatment-emergent adverse events (AEs) were mild (except 1 moderate AE [headache]). One participant had an AE leading to study drug discontinuation. There were no serious AEs or deaths.
ConclusionBased on results from this study, the proposed commercial and clinical formulations of ETR 2mg were bioequivalent in terms of rate and extent of absorption. No significant differences were demonstrated in ETR exposure for the proposed commercial formulation under fed and fasted conditions. All treatments were generally safe and well tolerated.
Fatigue is highly prevalent in patients with IBD independent of the disease status but treatment options remain limited. A potential mediator in the pathophysiology of fatigue is tryptophan (Trp), a precursor of serotonin. Recently, reduced serum Trp levels have been linked to fatigue in patients with clinically and endoscopically inactive IBD. The aim of the current study was to determine the effect of oral 5-hydroxytryptophan (5-HTP), the direct precursor of serotonin, supplementation on fatigue in patients with inactive IBD.
MethodsThis multicentre, randomized, double-blind, cross-over, placebo-controlled trial included fatigued patients with IBD in clinical and biochemical remission (CRP <10mg/L, calprotectin <250 mg/kg), treated with immunosuppressants and/or biologicals. Fatigue was assessed with the fatigue VAS (fVAS, range 0-10) and defined by a fVAS ≥5. Patients were treated in a cross-over manner with 100 mg 5-HTP or placebo bid for two consecutive periods of 8 weeks, without an intermediate washout period. The primary endpoint was the proportion of patients reaching a 20% reduction in fVAS after 8 weeks of intervention (week 8 versus week 0 and week 16 versus week 8). Secondary outcomes were changes in validated FACIT-F score, scores for depression and anxiety and changes in Trp metabolites. The effect of the intervention on the outcomes was evaluated by linear mixed modelling (LMM), with the intervention, period and intervention x period as fixed factors and study participant as random factor.
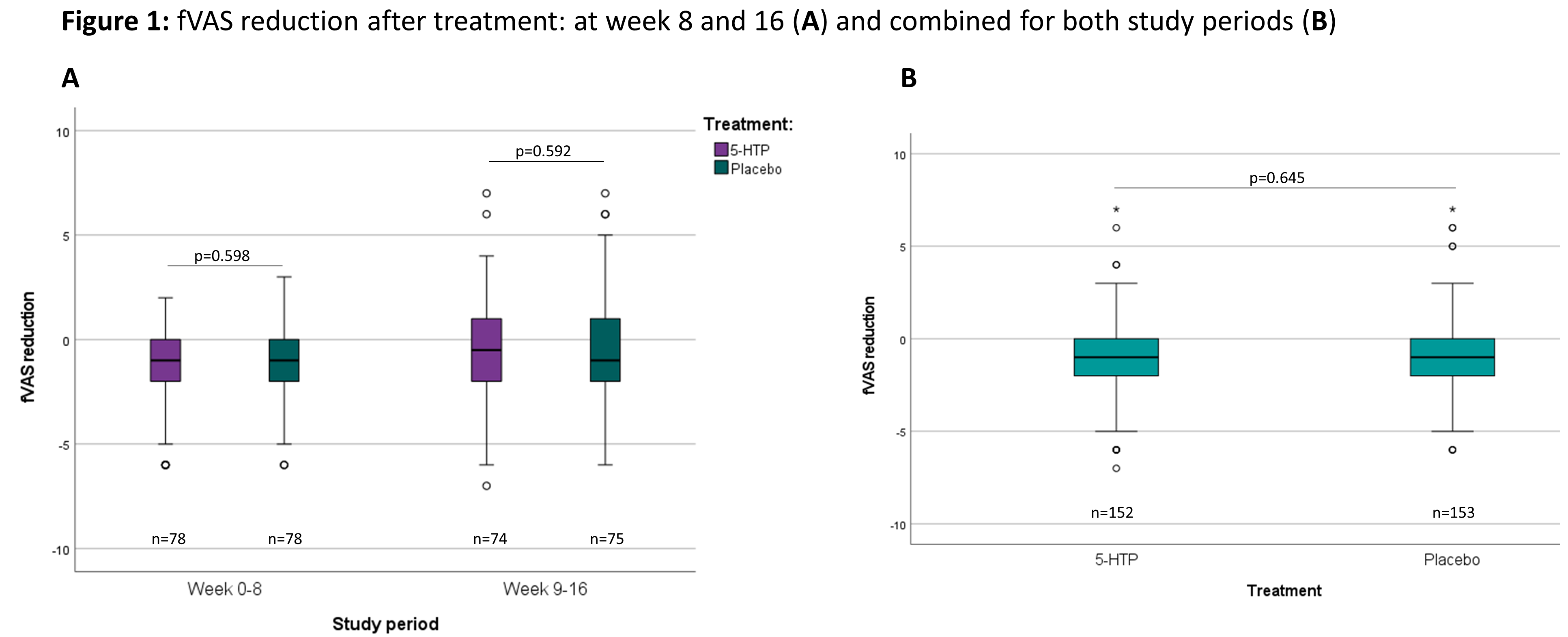
ResultsA total of 166 patients were included in 13 Belgian centres between December 2018 and November 2020 (baseline characteristics: Table 1). The dropout rate was 10.8%. The evolution of the fVAS throughout the study was comparable between both study groups and no difference was observed in fVAS reduction between placebo and 5-HTP (Figure 1). The proportion of patients reaching ≥20% reduction in fVAS did not differ between placebo (37.6%) and 5-HTP (35.6%) (p=0.830). The evolution of the other scores for fatigue, depression, anxiety and stress were also similar between placebo and 5-HTP (Table 2). A significant increase in 5-HTP and serotonin serum levels was observed during 5-HTP treatment compared to placebo; whereas serum levels of Trp and kynurenine were comparable. Globally, changes in fVAS were not associated with changes in those metabolites (Figure 2). Adverse events (AEs) were seen in 29.2% and 34.8% of patients under treatment with placebo and 5-HTP respectively (p=0.282).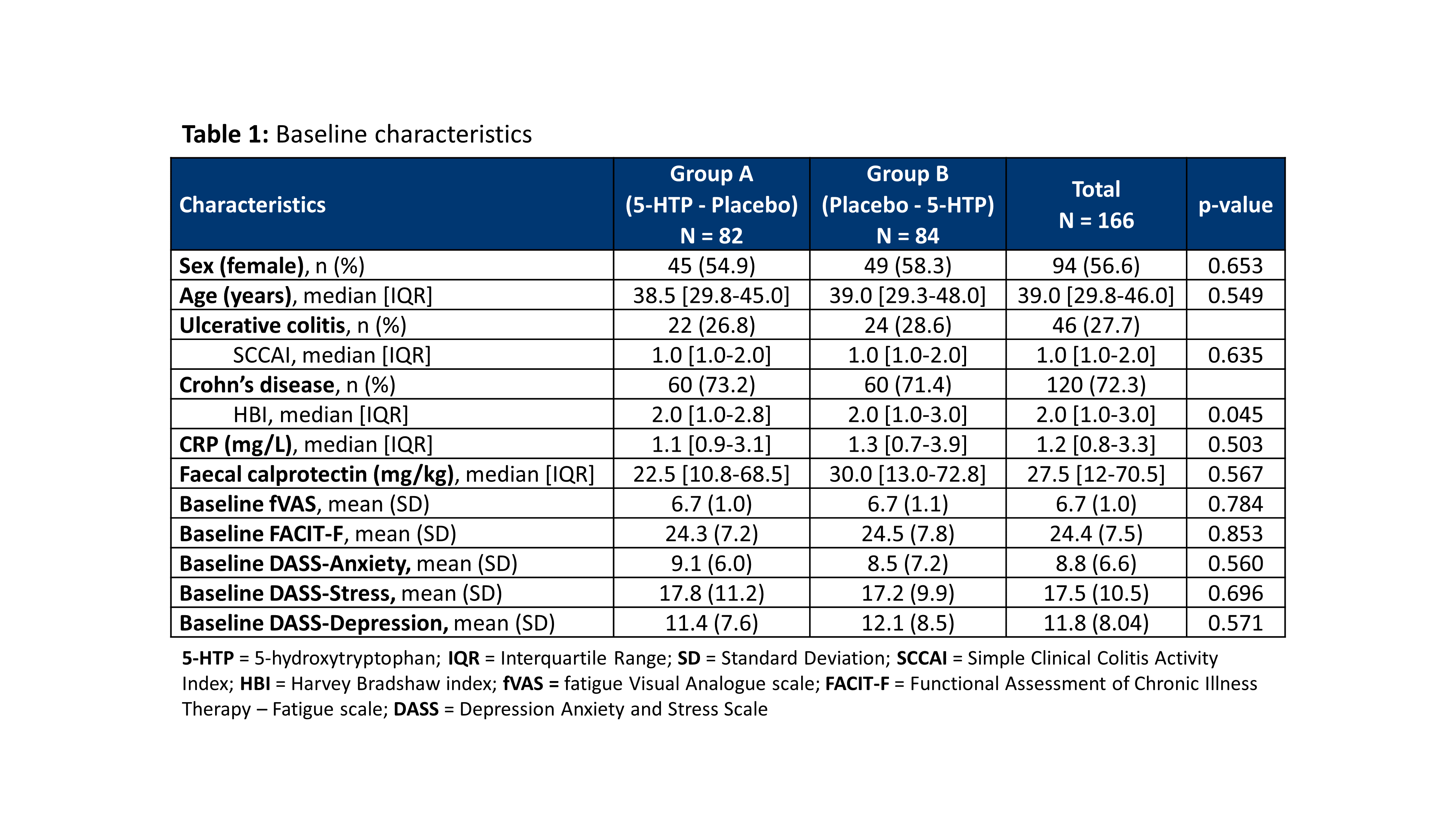


Despite a significant increase in serum 5-HTP and serotonin levels by oral treatment with 5-HTP, 5-HTP did not modulate IBD-related fatigue. Furthermore, treatment with 5-HTP had no impact on depression, anxiety and stress scores.
Background and Aims:
Inflammatory Bowel Disease (IBD) impacts the individuals’ quality of life and affects all family members considerably. Previous reviews have focused on the impact of IBD on the patient, with limited exploration of the impact of IBD on family members. Therefore, this review aims to synthesise existing knowledge on the impact of IBD on family members, their coping strategies, the support needed, and interventions for family members to prevent and alleviate the burden of IBD.
MethodsMethods:
A systematic review using the mixed-method systematic review approach suggested by Joanna Briggs Institute (JBI) and the Preferred Reporting Items for Systematic Reviews (PRISMA) was undertaken. A systematic search of six bibliographic databases: MEDLINE, EMBASE, PsycINFO, CINAHL, British Nursing Index, and Web of Science, was performed in February 2021. The search focused on the impact of IBD on family members and coping strategies and interventions for this population. A narrative synthesis was conducted.
Results:
In total, 3,258 records were identified, from which 33 relevant papers (2,748 participants) were included in the review, with case-control, cross-sectional, and qualitative designs. Synthesis of these papers found three themes: the impact of IBD on family members; the coping strategies for family members to overcome the negative impact of IBD; and the support needed. The IBD affects the family members in term of emotional well-being, fear and concern, relationship and social life, work and financial impacts, and leisure time and travelling. The coping strategy theme shows that family members use adaptive coping patterns such as acceptance, resilience, and emotional support from others. Maladaptive coping patterns such as denial following the initial relief of diagnosis, self-distraction, and self-blame were also used. In the theme ‘support needed’, family members reported the need for improved information about IBD, social support groups, self-help groups, educational meetings, and providing easy access to a counsellor or psychologist to support family members. There have been no studies assessing outcomes of interventions to relieve family members’ burden in the IBD population.
ConclusionConclusion:
Our findings suggest that policymakers in healthcare services should emphasise the multidisciplinary professional care model such as a family therapist, IBD nurse, and psychologist. Researchers could incorporate a bio-psycho-social approach into their work on IBD to improve quality of life of both patients and their family members.
1. To understand different kind of abstracts
2. To get knowledge about how to prepare an abstract
3. To get knowledge about general rules and guidelines concerning abstracts
4. To get knowledge about how to prepare a poster from an abstract
The efficacy and safety of risankizumab (RZB) as induction and maintenance therapy forpatients with moderately to severely active Crohn’s disease (CD) has been documented. Steroid-free clinical remission is an important additional treatment goal in CD. The efficacy of RZB by baseline steroid use during induction and steroid-free outcomes during maintenance was examined.
MethodsIn ADVANCE (NCT03105128) and MOTIVATE (NCT03104413), patients with moderately to severely active CD received intravenous (IV) RZB induction therapy or placebo (PBO) for 12 weeks. Patients with clinical response to RZB were re-randomised in a 52-week maintenance study (FORTIFY; NCT03105102) to subcutaneous (SC) RZB or PBO (ie, withdrawal). Patients receiving steroids (maximum prednisone or equivalent ≤ 20 mg/day; budesonide ≤ 9 mg/day) at baseline of the induction studies maintained stable doses for the 12-week study duration. A mandatory steroid taper per protocol was initiated at the beginning of maintenance for patients receiving steroids during induction. Patients losing clinical response (per investigator assessment) after initiation of taper could have their steroid dose increased up to the induction baseline dose. This analysis included patients who received RZB 600 mg IV in ADVANCE or MOTIVATE and patients who received RZB 360 mg SC in FORTIFY. Endpoints reported included clinical remission (defined by CD Activity Index [CDAI] or stool frequency/abdominal pain score [SF/APS] criteria) at week 12 of induction by baseline steroid use, steroid-free clinical remission (CDAI or SF/APS), steroid-free endoscopic response, and steroid-free endoscopic remission at week 52 of maintenance. Steroid discontinuation rates over 52 weeks of maintenance were also assessed.
ResultsInduction of clinical remission at week 12 with RZB 600 mg IV in ADVANCE or MOTIVATE was independent from baseline steroid use (Figure 1).Clinical remission rates (CDAI or SF/APS) at week 12 were similar for patients using steroids vs those who were not (33.8%–42.0% vs 34.9%–46.6%; Figure 1). Steroid use decreased over time in FORTIFY, with a greater proportion of patients receiving RZB 360 mg SC discontinuing steroids at week 52 vs withdrawal (PBO SC; Figure 2A). Rates of steroid-free clinical remission (P ≤ .012), steroid-free endoscopic response (P < .001), and steroid-free endoscopic remission (P < .001) were significantly higher with RZB 360 mg SC vs withdrawal (PBO SC) at week 52 of maintenance (Figure 2B–2C).

Efficacy of RZB induction therapy was independent of baseline steroid use. RZB maintenance promotes high rates of steroid-free clinical and endoscopic outcomes, demonstrating a benefit of RZB treatment in CD.
1. To understand adherence in a broad perspective
2. To review existing knowledge on adherence
3. To emphasize possible predictors for low adherence
4. To provide with some tools to measure adherence
Although characteristic findings within the mesentery have been described early on by Burrill Crohn himself, the role within Crohn’s disease has only partially been deciphered yet. Thus, this tandem talk will address the following three central points in the field:
1) Why this topic is of interest.
2) Reasons that suggest a protective role of the mesentery.
3) Reasons that suggest a disease-driving role of the mesentery.
This will be done by jointly discussing the available experimental, translational as well as surgical evidence. We will conclude by identifying the gaps in knowledge and by highlighting the ongoing clinical trials in the field.
1. to give a surgical point of view concerning adipose tissue and Crohn's disease
2. to present the reasons that underline a protective side of the mesentery, with the recent good results with KONO-S anastomosis (preservation of the mesentery) and stricturoplasty
3. to have an overview of the reasons that underline a disease-driven role of adipose tissue, with good results obtain with mesorectal resection during abdominoperineal resection for CD in Amsterdam and good preliminary results obtain by Coffey in Ireland with ileocecal resection with mesenteric resection for CD
4. to give an overview of the randomized trials in progress on mesenteric resection during ileocecal resection for Crohn's disease



