Welcome to the e-CCO Library!
DOP019
Efficacy of autologous haematopoietic stem cell transplantation for refractory Crohn’s disease
A. López García1, M. Rovira2, A. Jauregui Amezaga1, P. Marin3, A. Salas1, S. Pinó Donnay1, R. Barastegui1, F. Feu1, J. Elizalde1, F. Fernández-Avilés2, C. Martínez4, G. Gutierrez2, L. Rosiñol2, E. Carreras2, A. Urbano2, M. Lozano3, J. Cid3, M. Suárez-Lledó2, J. Mensa5, J. Rimola6, S. Rodriguez6, M. C. Masamunt1, D. Comas1, A. Ramirez Morros1, M. Gallego1, I. Ordás1, J. Panés1, E. Ricart*1
1Hospital Clinic Barcelona, Gastroenterology, Barcelona, Spain, 2Hospital Clinic Barcelona, Haematology, Barcelona, Spain, 3Hospital Clinic Barcelona, Biomedic Diagnostic Centre, Barcelona, Spain, 4Hospital Clinic Barcelona, Ginecology, Barcelona, Spain, 5Hospital Clinic Barcelona, Internal Medicine, Barcelona, Spain, 6Hospital Clinic Barcelona, Radiology, Barcelona, Spain
DOP020
Prediction of clinical and endoscopic remission after autologous stem cell transplantation in treatment refractory Crohn’s disease: pooled results from the ASTIC trial
J. Lindsay*1, M. Allez2, M. Clark3, M. Labopin4, E. Ricart5, G. Rogler6, M. Rovira7, J. Satsangi8, D. Farge9, C. Hawkey3
1Blizard Institute, Barts and the London School of Medicine, Centre for Immunobiology, London, United Kingdom, 2APHP St. Antoine Hospital, Department of Gastroenterology, Paris, France, 3Nottingham Digestive Diseases Centre, Department of Digestive Diseases, Biomedical Research Unit, Nottingham, United Kingdom, 4European Group for Blood and Marrow Transplantation (EBMT), Paris, France, 5Hospital Clinic Barcelona, Gastroenterology, Barcelona, Spain, 6University Hospital Zürich, Department of Gastroenterology and Hepatology, Zürich, Switzerland, 7Hospital Clinic de Barcelona, Department of Haematology, Barcelona, Spain, 8Western General Hospital, Gastrointestinal Unit, Edinburgh, United Kingdom, 9Hospital Saint-Louis, Department of Internal Medicine and Vascular Pathology, Inserm U 976, Paris, France
DOP021
Long-term efficacy of autologous haematopoietic stem cells transplantation for refractory Crohn’s disease: 10 years of Milan experience without CD34+ cell selection
A. Cassinotti*1, F. Onida2, C. Annaloro2, G. Saporiti2, M. Fichera1, M. Daperno3, B. Motta2, P. Fociani4, E. Tagliaferri2, G. Sampietro5, D. Vincenti2, A. Gregorini2, G. Maconi1, M. Nebuloni6, A. Cortelezzi2, S. Ardizzone1
1Gastroenterology Unit, Luigi Sacco University Hospital, Milan, Italy, 2Bone Marrow Transplantation Centre, Fondazione Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, University of Milan, Milan, Italy, 3Gastroenterology Unit, AO Ordine Mauriziano, Turin, Italy, 4Luigi Sacco University Hospital, Pathology Unit, Milan, Italy, 52nd Division of Surgery, Luigi Sacco University Hospital, Milan, Italy, 6Pathology Unit, Luigi Sacco University Hospital, Milan, Italy
DOP022
Targeting immune cell metabolism: LYC-30937, a novel therapeutic approach for inflammatory bowel disease
L. Carter1, R. Morgan1, C. Lesch1, M. Spahr1, L. Franchi2, I. Monteleone3, G. Monteleone3, G. Glick2, H. J. Wilkins1, P. Higgins*2
1Lycera, Ann Arbor, Michigan, United States, 2University of Michigan, Ann Arbor, Michigan, United States, 3Tor Vergata, Rome, Italy
DOP023
Safety and efficacy of a novel IV targeted pegylated liposomal prednisolone product (Nanocort): results from a phase 2a study in patients with active ulcerative colitis
G. van Assche1, P. Rutgeerts1, M. Ferrante1, M. Noman1, H. Fidder2, B. Oldenburg2, J. Metselaar3, 4, S. Vermeire*1
1University Hospitals Leuven, Department of Gastroenterology, Leuven, Belgium, 2University Medical Centre Utrecht, Department of Gastroenterology and Hepatology, Utrecht, Netherlands, 3Enceladus Pharmaceuticals, Naarden, Netherlands, 4University Clinic RWTH, Experimental Molecular Imaging, Aachen, Germany
Ulcerative Colitis (UC) is a chronic, relapsing inflammatory disease affecting the mucosal lining of the rectum and the colon to a variable extent. Corticosteroids have long been a cornerstone in the treatment of UC, despite considerable side effects including Cushingoid facies and weight gain, acne, hyperglycaemia, insomnia, infections, and osteoporosis after extended use. Nanocort is a novel pharmaceutical composed of prednisolone sodium phosphate enclosed in 100 nm PEGylated liposomes, which, after IV infusion, selectively target and accumulate in inflamed bowel lesions and selectively deliver high and effective concentrations of corticosteroids, thus reducing the required total steroid dose and dosing frequency.
In an exploratory, 2-centre (neoplastic lesions [NL] and BE), randomised, placebo-controlled, observer-blind phase 2a study, the safety and efficacy of Nanocort was evaluated in 18 patients aged 22–63 with moderate-to-severe active UC. Two IV doses of 150 mg Nanocort (n = 14) or saline (n = 4) were given as slow infusions 2 weeks apart. Overall safety (primary endpoint), pharmacokinetics, and efficacy were assessed at weeks 2, 4, 8, and 12 after start of treatment.
The median total Mayo score (tMS, 0–12) at baseline was 10 (range 7–12). At week 4 the Nanocort group showed clear benefit in 70% of the patients with 4 patients out of 13 in remission. Further, 7 out of 13 patients showed a reduction of the endoscopy sub score of ≥ 1 point reaching ≤ 1 point. Rapid effects on partial Mayo score (pMS, 0–9) were shown with persisting remissions in 7/13 patients treated with Nanocort. Remission was defined as a post-treatment MS of ≤ 2 points with all sub scores ≤ 1 point. Typical steroid-related adverse effects were sparse with isolated mild to moderate cases of acne, dyspepsia, nausea, and gastritis. No significant suppression of urinary cortisol was found, nor were there any indications of hyperglycaemia. Some patients experienced infusion reactions probably related to the trial medication. One patient experienced an exacerbation of psoriasis upon withdrawal.
The results of this phase 2a study demonstrate that IV Nanocort can be a safe new therapy with fast and durable therapeutic benefit for patients with active UC without the drawbacks of oral steroid standard-of-care. Larger studies are warranted.
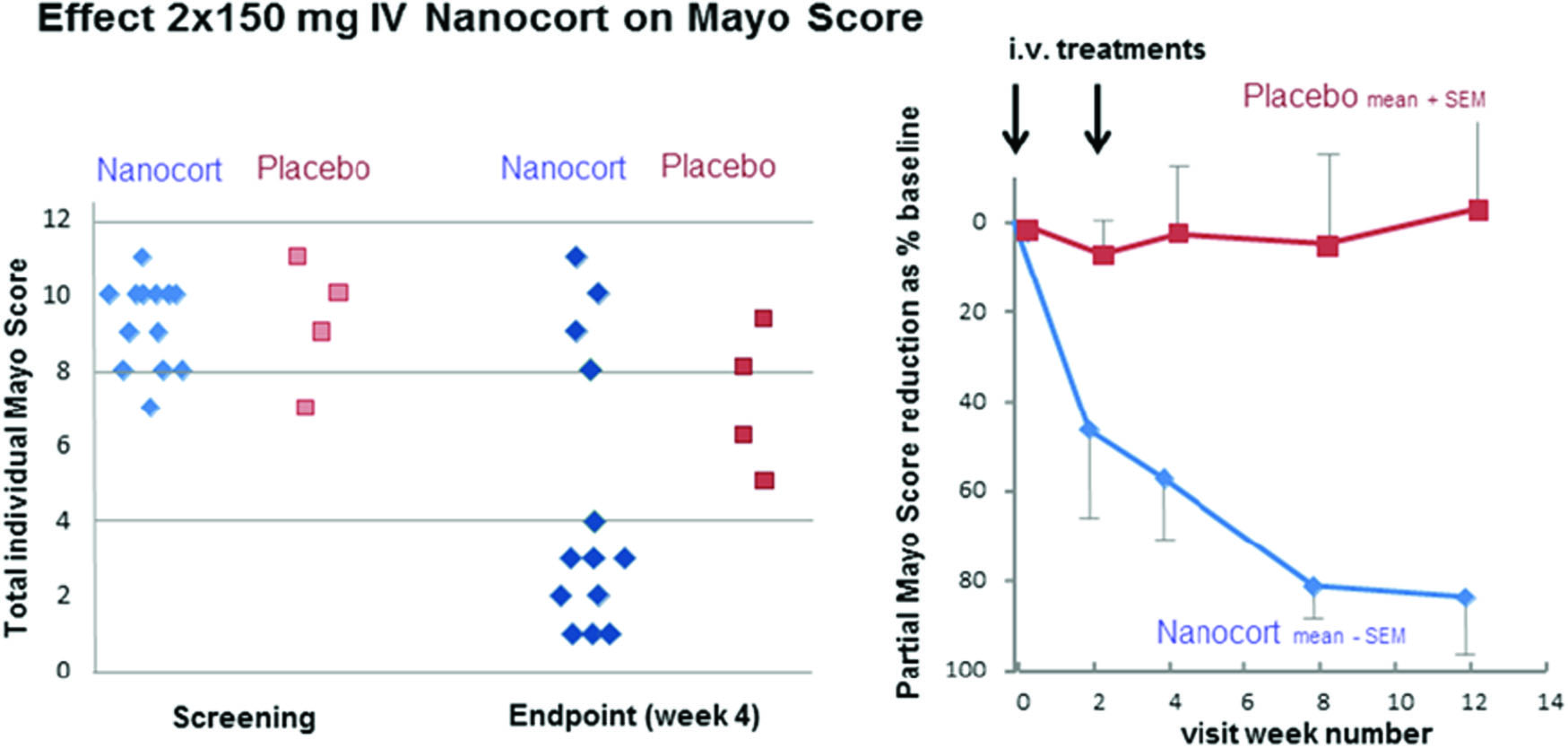
Figure 1. Nanocort effect.
DOP024
Electrical vagus nerve stimulation as an innovative treatment in inflammatory bowel diseases
V. Sinniger1, 2, 3, S. Pellissier3, 4, D. Hoffmann5, C. Trocmé6, L. Vercueil3, 7, D. Clarençon3, 8, B. Bonaz*3, 9
1Hospital University Centre, Hepatogastroenterology, Grenoble Cx9, France, 2Hospital University Centre, Gastroenterology, Grenoble Cx9, France, 3Inserm U836, Grenoble, France, 4University of savoie Mont Blanc, Psychology department, chambéry, France, 5Hospital University Centre, Neurosurgery, Grenoble, France, 6Hospital University Centre, Biology, Grenoble, France, 7Hospital University Centre, Neurology, Grenoble Cx9, France, 8Research Institute of the French Army, Brétigny-sur-Orge, France, 9Hospital University Centre, Gastroenterology, Grenoble, France
DOP025
Clinical response to anti-MMP9 antibody (GS-5745) is accompanied by histologic improvement in ulcerative colitis
W. Sandborn1, 2, B. Bhandari3, R. Fogel4, J. Onken5, E. Yen6, E. Huntzicker6, Y. Xin6, D. French6, J. Silverman6, B. Kanwar6, M. Subramanian6, J. McHutchison6, S. Lee7, L. Shackelton8, L. Stitt8, R. Pai9, B. Levesque*2, G. D’Haens10, 11, B. Feagan8, 12
1University of California, San Diego, California, United States, 2Robarts Clinical Trials, San Diego, California, United States, 3Delta Research Partners, Monroe, Louisiana, United States, 4Clinical Research Institute of Michigan, LLC, Chesterfield, Michigan, United States, 5Duke University Medical Centre, Durham, North Carolina, United States, 6Gilead Sciences, Inc, Foster City, California, United States, 7University of Washington, Seattle, Washington, United States, 8Robarts Clinical Trials, London, Ontario, Canada, 9Mayo Clinic Arizona, Scottsdale, Arizona, United States, 10Academic Medical Centre, Amsterdam, Canada, 11Robarts Clinical Trials, Amsterdam, Netherlands, 12University of Western Ontario, London, Ontario, Canada
DOP026
The Toll-like-receptor 9 agonist DIMS0150 demonstrates therapeutic efficacy for the patient-reported outcome measures PRO-2 and ClinPRO in moderate-to-severe active ulcerative colitis
R. Atreya*1, S. Bloom2, F. Scaldaferi3, V. Gerardi4, C. Admyre5, A. Karlsson5, T. Knittel5, J. Kowalski5, M. Lukas6, R. Löfberg7, R. Petryka8, R. Schnabel9, U. Seidler10, S. Nancey11, M. Neurath1, C. Hawkey12
1University of Erlangen-Nuernberg, Department of Medicine 1, Erlangen, Germany, 2University College London Hospital, Department of Gastroenterology, London, United Kingdom, 3Catholic University of Rome, Internal Medicine Department / Gastroenterology Division, Rome, Italy, 4Catholic University of Rome, Rome, Italy, 5Index Pharmaceuticals, Stockholm, Sweden, 6IBD Clinical and Research Centre, Clinical Centre Isacre Lighthouse, Prague, Czech Republic, 7Karolinska Institute and Sophiahemmet, Stockholm, Sweden, 88NZOZ Vivamed, Warsaw, Poland, 9Pannonia Maganorvosi Centrum, Budapest, Hungary, 10MHH, Department of Gastroenterology, Hepatology, and Endocrinology, Hannover, Germany, 11Lyon-Sud Hospital, Department of Gastroenterology, Lyon, France, 12Nottingham University Hospitals, Department of Gastroenterology, Nottingham, United Kingdom